
While the multiple attributes method gains ground and traditional lab methods improve, broad efforts are underway to determine biopharmaceuticals’ most significant critical quality attributes and enable real-time release.

While the multiple attributes method gains ground and traditional lab methods improve, broad efforts are underway to determine biopharmaceuticals’ most significant critical quality attributes and enable real-time release.

The Quartic Platform is a new, AI-powered smart manufacturing platform that can be integrated into legacy facilities and manufacturing processes.

Technical advances in process understanding and control must be accompanied by a change in mindset.

The GE Healthcare and Rockwell Automation collaboration will help meet the needs of the biopharma 4.0 era.

As automation in biomanufacturing becomes more important, so does the need to integrate process data.

The companies partnered to build a 500-L single-use pilot-scale plant for biologics production.

Applied throughout a product’s lifecycle and across a company’s portfolio, quality risk management and knowledge management will enable more agile manufacturing and better quality standards in the future.
Non-destructive surface area measurement can improve stability testing.

New software tools by Tecan, a provider of automated laboratory instruments and solutions, complement each other to enhance process monitoring of its liquid-handling platforms.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.

FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

Modern technologies, including Industry 4.0 and the Industrial Internet of Things, offer opportunities to increase biopharmaceutical manufacturing efficiency.
Late-stage and commercial biomanufacturing pose a challenge to cell-culture processing.

Single-use technologies have become increasingly prevalent in final fill/finish operations for biologics.

Siemens will become a preferred supplier for Sartorius Stedim Biotech’s automation solutions, and SSB will create a globally standardized automation platform for its biopharmaceutical manufacturing systems.

ABB’s modeling software, Shop Floor Integration V2.0, allows engineers to validate the connection of existing pharmaceutical manufacturing equipment with Werum’s PAS-X manufacturing execution system in a simulation prior to running it live.

The company will provide the first FlexFactory manufacturing platform for cell therapy manufacturing.

Aseptic fill/finish is crucial in biopharma manufacturing and is optimized through automated technology.

InstantGMP INV is a validated software for real-time material tracking and inventory control in biopharmaceutical manufacturing.

The need to improve and understand processes is moving PAT and more advanced control strategies beyond the lab into manufacturing and downstream applications.

More published data and initial regulatory approvals are needed to drive adoption of continuous bio-manufacturing.
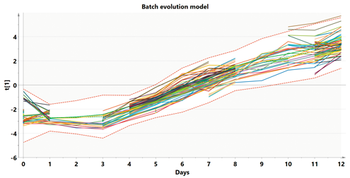
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

GE Healthcare will equip Cellular Biomedicine Group's cell therapy manufacturing facility in Shanghai, China, with its FlexFactory single-use platform, designed to speed up cell therapy manufacturing timelines.

The IFPAC annual meeting on advancing the understanding and control of manufacturing processes using process analytical technology will be held Feb. 11-14, 2018.