
The company’s new modules offer scalable single-pass diafiltration and were exclusively showcased during its Leadership Forum series in Westborough, MA.

The company’s new modules offer scalable single-pass diafiltration and were exclusively showcased during its Leadership Forum series in Westborough, MA.

CPhI Pharma Awards honor companies and individuals driving the pharma industry forward.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.
Consider automation early in the rollout of clinical translation and scale up of clinical-trial protocols.

The International Society for Pharmaceutical Engineering announced the release of ISPE GAMP Good Practice Guide: IT Infrastructure Control and Compliance (Second Edition).

The International Society of Automation (ISA) and Siemens entered a global partnership to increase awareness of industrial cybersecurity needs and standards.

IDBS described benefits and best practices for laboratory electronic data systems.

The annual meeting of the Honeywell Users Group for the Americas will discuss industrial automation.

An influx of millennial workers may have an impact on whether pharma manufacturers choose to implement IIoT technology.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.
Process controls get some upgrades to better reflect real-time conditions.


Miniature bioreactors add value by reducing validation efforts.

This case study reviews how quality-by-design principles can be implemented in an intermediate chromatography purification step that uses cation-exchange chromatography.Abstract

Sorendls/Getty ImagesTo maintain a state of control and comply with regulatory authorities, many pharmaceutical, biotech, and medical-device companies have adopted continued pro

The European Pharmacopoeia has incorporated new provisions to encourage the use of in-process quality control methods.

This article presents first-hand perspectives from industry users to suppliers of single-use sensors.

MedicalRF.com/Science Photo Library-ANDRZEJ WOJCICKI/Henrik Jonsson/Stocktrek Images; Dan Ward

FDA provides recommendations for submitting analytical procedures and methods validation data.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.
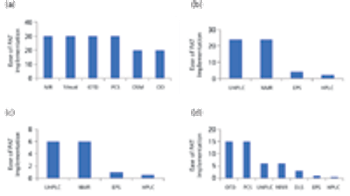
The authors review the various analytical methods that can enable use of PAT.