
To ensure the sterility of parenteral biopharmaceutical products, it is necessary to employ certain tools, technologies, and standard operating procedures.

To ensure the sterility of parenteral biopharmaceutical products, it is necessary to employ certain tools, technologies, and standard operating procedures.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.

Along with particulate control and determination, speakers at the June conference in Rockville, Maryland, examined the role of protein aggregation and immunogenicity
Protecting against microbiological contaminationover the whole manufacturing process grows increasingly important.

This article reviews systems and processes that enable a laboratory to approach troubleshooting in an effective way, while also taking a proactive, preventive approach to managing atypical laboratory scenarios.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to handle audits and inspections during business expansion.

Biopharma must see regulators as partners in their efforts to provide safe and effective therapies.
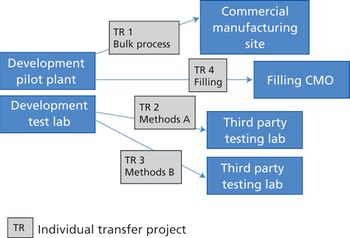
Careful planning, adequate staffing levels, and experienced project managers can help avoid pitfalls of transferring processes from one facility to another.
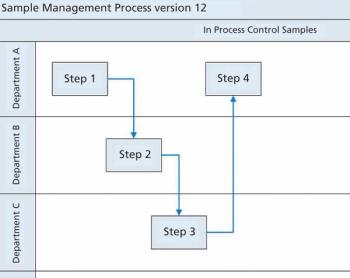
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

Process maps and risk assessments are among the valuable tools operators can apply to reduce the risk of microbial contamination.
Enterprise quality management systems can help shift the quality emphasis from corrective to preventive actions.
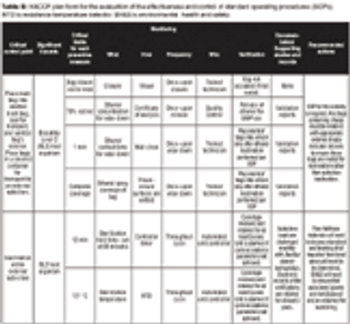
A Risk-Management Case Study.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.

SOPs are written job aids that detail the procedure of how to do a specific job task correctly.