While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.
Randi Hernandez was science editor at BioPharm International from September 2014 to May 2017.

While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

Single-use bags containing toxic or hazardous materials required special handling.
The success of a truly integrated continuous processing platform relies on the collaborative efforts of upstream and downstream specialists.

BioPharm International speaks with a CMO to find out how it is coping with capacity challenges, regulatory authority scrutiny, supply chain reliability, and new requests from clients to incorporate continuous manufacturing into processing lines.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?

Improving the bioreactor growth environment increases the rigor of bioprocessing runs.

The number of deficiencies found in foreign and UK-based facilities increased in 2016.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

The mAb is the first approved treatment that targets the progressive form of the disease.

Richard D. Braatz, PhD, will discuss using mathematical models to design a continuous drug manufacturing plant and the differences between batch and continuous operations for biologics.

A blog posted on Health Affairs on March 7, 2017 presents a study that tested PhRMA’s long-standing argument that high prices for drugs fund research and development in the pharmaceutical industry.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

In a press release, Momenta announced on Feb. 16, 2017 that Momenta/Sandoz’s fill/finish contract manufacturer, Pfizer, received a warning letter for the manufacture of the 40 mg of Glatopa (glatiramer acetate injection), Momenta’s generic version of the drug Copaxone. Pfizer said in the statement that the warning letter does not affect the production or shipment of the 20 mg version of the drug, which is already approved by FDA.

The regulatory agency rejected the medication, citing various issues related to device use.

PhRMA submits comments to the The Office of the United States Trade Representative encouraging protection of US innovation in foreign markets.
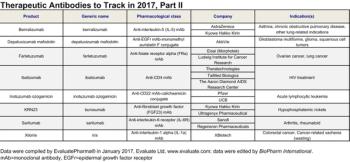
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

On a recent call, Catalent revealed that it has reached more than 90% of its current capacity and discussed how tax policy changes could affect the outsourcing industry.

Aggressive petitioning by ViroPharma kept a generic equivalent to Vancocin off the market for more than two years.

According to results from the FOURIER trial, Repatha significantly reduced the risk of cardiovascular events and death in patients with atherosclerotic cardiovascular disease.

Patients with relapsing-remitting multiple sclerosis (MS) who are treated with currently available disease-modifying drugs (DMARDs) usually experience disease reactivation within the first five years of treatment follow-up. Many of the available treatments for MS only confer complete control of disease activity in a small percentage of patients.

Pfizer executives were not among the pharma representatives who met with Trump yesterday to discuss various issues affecting the industry, a circumstance that Pfizer attributed to a schedule conflict with its quarterly earnings call. Despite the company’s absence from the meeting, Pfizer’s CEO Ian C. Read commented on some of Trump’s policy suggestions, and also discussed sales trends and projections for Pfizer’s R&D pipeline.

Trump met with pharma manufacturers and makes a statement focused on domestic manufacturing, FDA approvals, and drug pricing.

Operational improvements at Pall contributed to the overall growth in the Danaher life-sciences sector.

Despite the threat of competition, both from biosimilars to Humira and new therapies that work through other mechanisms of action, AbbVie says Humira is still number one.

Alexandre Juillerat, PhD, innovation senior scientist at Cellectis, discusses novel construct UCART123, an investigational agent that is on deck to be the first gene-edited T-cell product in the United States.

The company will gain the rights to a mutein that is believed to help maintain immune system homeostasis.

Researchers from Cellectis investigate how external signals in the tumor microenvironment could control the cell-surface expression and specificity of engineered CARs.

In a recent deal with the Federal Trade Commission, Endo agreed to refrain from entering into future pay-for-delay patent settlements for ten years.

Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

Published: October 30th 2014 | Updated:

Published: October 3rd 2014 | Updated:

Published: October 28th 2014 | Updated:

Published: November 7th 2014 | Updated:

Published: September 30th 2014 | Updated:

Published: October 1st 2014 | Updated: