
Catalent has unveiled investment plans to create a European center of excellence for clinical biologics formulation development and drug product fill/finish services.

Catalent has unveiled investment plans to create a European center of excellence for clinical biologics formulation development and drug product fill/finish services.

The acquisition will give the CDMO additional clinical filling options in Europe, which is expected to come online in 2021.

The COVID-19 pandemic has created a rise in demand for R&D and a shift in focus for some contract organizations.

Vibalogics is contracted to manufacture Janssen's COVID-19 candidate vaccine at its GMP-accredited facility in Cuxhaven, Germany.

Fujifilm allocates production volumes for COVID-19 treatments in 2021 at its Denmark facility.

Technology and capacity investments create opportunities in the cell and gene therapy arena for CDMOs and biopharma alike.

CDMOs and CMOs will continue to invest in biopharmaceutical services and facilities as the bio/pharmaceutical industry looks to biosimilars and personalized medicine.

Catalent completes purchase of biologics fill-finish and oral solid dose facility in Anagni, Italy

New training facilities, laboratories, packaging, gene therapy manufacturing, and biologics manufacturing highlight Thermo Fisher Scientific expansions.

Catalent recently held a groundbreaking ceremony at its Bloomington, Indiana pharmaceutical fill/finish site.

Lyophilization Services of New England acquired a sterile injectables manufacturing facility in León, Spain.

The Massachusetts site, formerly associated with an affiliate of Shire, is Rentschler Biopharma’s first facility in the US.

The maturation of single-use technologies presents commercial bioprocessing options for small-volume drug products.

Pharmaceutical scientist association announces upcoming term’s board of directors.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.

This article highlights 15 years of changes in biopharmaceutical manufacturing.

In adding a Vanrx Pharmasystems aseptic filling isolator, FUJIFILM adds fill/finish for gene therapies and viral vaccines.

Anthony Qu, PhD, vice president of Scientific Affairs at Halo Pharma, will give a presentation on fixed-dose combination products, drug products containing multiple active ingredients, as an effective approach for simplified dosing at CPhI North America on Wednesday, April 25, 2018 in Philadelphia, PA.
A different perspective on controlling fixed costs of biomanufacturing, based on know-how from other industries, provides a competitive edge, says the CEO of Samsung BioLogics.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.

Paragon Bioservices will build a new cGMP facility in Maryland and expand its existing cGMP facility at the University of Maryland's BioPark.

Contract manufacturers await a promising pipeline of drug products to jump-start stagnant growth.
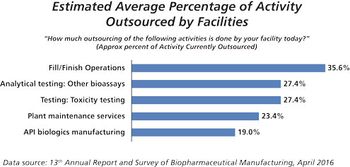
This key bioprocessing segment is expecting continued growth.
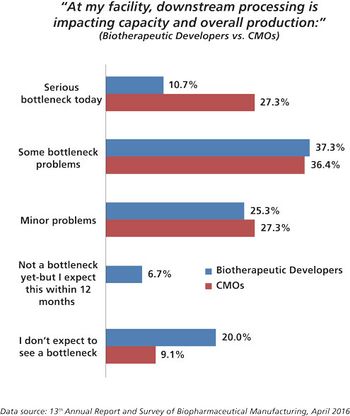
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.