
Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

A new probe developed by researchers at the University of Melbourne, Australia, would allow NMR to be used without the use of microwaves, and with smaller machines.
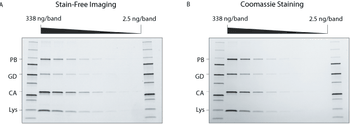
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.

Researchers describe a new method to compare the higher-order structure of a reference biologic with its proposed biosimilar product candidates.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.
USP evaluates raw materials used in the chemical synthesis of peptides.
Advances in instrumentation, software, and methodologies provide more information than ever on the higher-order structure of proteins.
Review the importance of characterization studies during biosimilars development and related analytical methods.

At Pittcon 2013, Bruker introduced a range of instruments and software updates including two LC-triple quadrupole (LC-TQ) mass spectrometers.

As biopharmaceutical/pharmaceutical companies increase their development of biologic-based drugs, companies providing analytical instrumentation and laboratory testing goods and services are, in turn, offering improved tools for biologic characterization, biomanufacturing, and related testing.
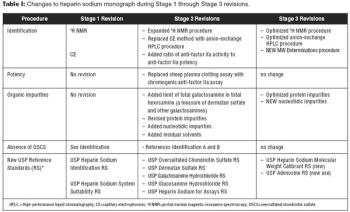
USP optimizes identification tests and impurities procedures.
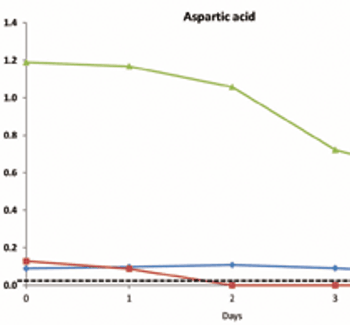
Current expectations in bioprocessing and a framework for using NMR to enhance a QbD approach.
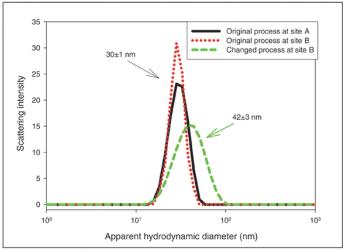
Spectroscopic methods such as circular dichroism can detect subtle differences in higher order structure before and after changes in process and formulation.
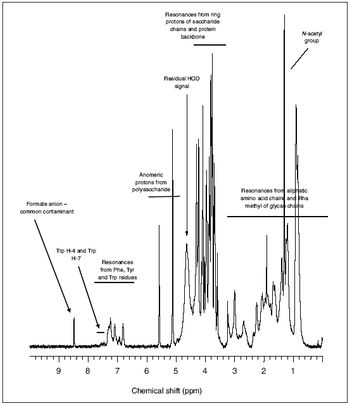
Simple methods can characterize polysaccharide vaccines and recombinant cytokines at high resolution.
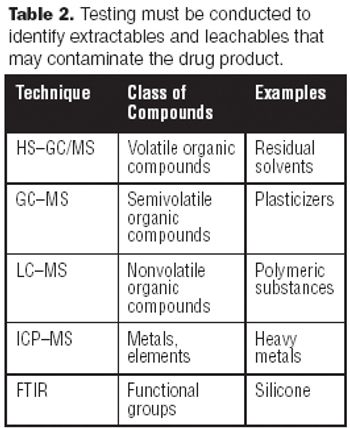
A tremendous amount of analytical testing is required to support a biopharmaceutical product from discovery, development, and clinical trials, through manufacturing and marketing. Numerous methods are used to fully characterize large molecules because of their complexity-characterizing them is significantly more difficult than it is for small molecules. Biopharmaceuticals are produced via living systems, i.e., E. coli, yeast, or mammalian cells, which require additional testing matrices.
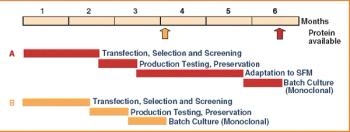
Over the last three decades, numerous protein expression systems have been developed with various quality requirements on large and small scales. Huge steps have been made in large-scale protein production in mammalian systems while the small-scale mammalian systems are expensive and inflexible. Thus, small-scale production is done in simpler expression systems, sometimes sacrificing the quality of the proteins. However, relief is on the way.