
Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.

Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

The investment builds on a collaboration the companies entered into in 2007 for various biomanufacturing projects.

GE’s new facility, which will be operational in 2019, will produce a fiber-based chromatography platform for more efficient biopharmaceutical purification.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.

The media, by Tosoh Bioscience, is composed of calcium and phosphate buffers and offers mixed-mode properties for biomolecule purification.

The new resin used a combination of “jetting” technology and a high-performance Protein A ligand.

More published data and initial regulatory approvals are needed to drive adoption of continuous bio-manufacturing.
Process understanding and careful assessment of risks are essential in developing viral clearance programs.

Early adopters are benefiting from lower costs and increased productivity.

The Wilden Quattroflow QF10k size pump from PSG, a Dover company, has been designed to fill the gap between the existing QF4400/5050 and QF20k pump sizes.
Advances in TFF and single-use systems help advance UF/DF on the continuous processing path.

The Quattroflow QF10k size pump from PSG, a Dover company, has been added to its line of quaternary, four-piston diaphragm pumps.

Single-use systems provide replaceable fluid paths.

The acquisition gives GE Healthcare access to a nanofiber-based platform purification technology that can offer improvements in biopharmaceutical productivity.
This article discusses the potential of using high-productivity membrane chromatography to achieve intensified and flexible virus manufacturing.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.

This study outlines methods for an alternative protein-polishing process for challenging proteins.
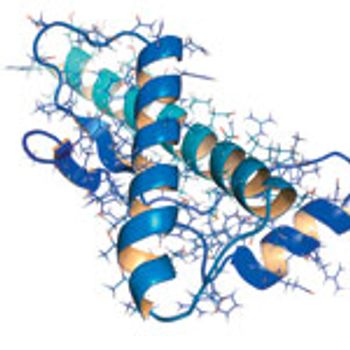
. This study is an attempt to produce a fusion protein by binding the fragment NT-gp96 in upstream of sequence of the N terminal fragment (NT300) of the NS5B gene in an expression vector.
A case study demonstrates that affinity chromatography can offer efficiency and scalability for gene therapy manufacturing using viral vectors.

Single-use and single-pass TFF devices are facilitating advances in biopharma manufacturing.