
Advances in wearable devices have made it possible to deliver high-volume, high-viscosity biologics.

Advances in wearable devices have made it possible to deliver high-volume, high-viscosity biologics.

Sensors and devices being developed by nGageIT Digital Health Solutions can track patient use of oral solid-dosage or injectable drugs.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

Researchers at the University of Illinois developed a method to transplant pancreatic islet cells from pigs more easily to treat type I diabetes.

The acquisition strengthens an already existing collaboration between the companies to advance clinical development of therapies across multiple drug categories.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.

ADC Bio experts warn of impending problems in the ADC pipeline with millions wasted in development costs.

The EMA’s Committee for Medicinal Products for Human Use has recommended marketing authorization approval for AstraZeneca’s benralizumab, a monoclonal antibody for treating severe asthma.

The authors of the study believe it could have significant implications for the discovery of new dermatological products for major diseases such as psoriasis.

The company has officially opened a local brand office in South Korean to support its existing business in the region.
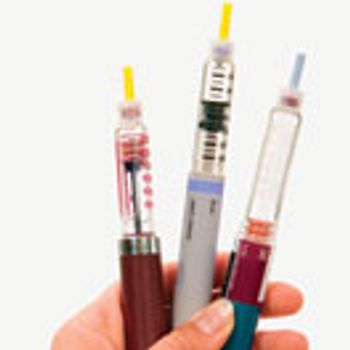
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

The $72-million investment, part of a larger $850-million investment into its US operations, will allow the drugmaker to replace an outdated insulin vial-filling line and to upgrade technology at its Indianapolis manufacturing plant.

Through the support of the Japanese agency, Daiichi Sankyo intends to further develop its genetic vaccine platform focused around its new nucleic acid delivery technology.

Gelest, a manufacturer and provider of silane, silicone, and metal-organic compounds, released a range of dual-function poly(ethylene glycol) (PEG) reagents that enable new approaches to PEGylation for bioconjugates.

Virgin Atlantic Cargo and Delta Cargo have opened a new Pharma Zone at their joint facility at London Heathrow Airport for the handling and storage of pharmaceutical shipments.

There is a lot of interest in delivering biologics via non-invasive routes in attempt to improve patient compliance and convenience.

Nemera is now authorized to handle, assemble, sterilize, and store pharmaceutical drugs and medicinal products for autoinjector combination products at its facility in Neuenburg, Germany.

UPS has revealed a new system to improve drug supply chain security, in compliance with the Drug Supply Chain Security Act (DSCSA).
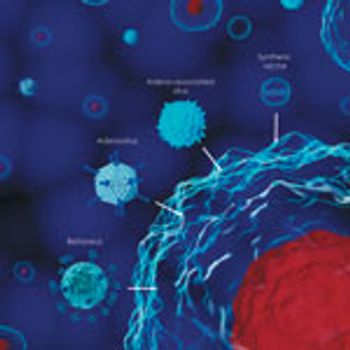
A case study demonstrates that affinity chromatography can offer efficiency and scalability for gene therapy manufacturing using viral vectors.

This review examines how microfluidics has been used in the formulation, preclinical, and clinical development of gene-delivery nanoparticles.

Genentech gets priority review for its application seeking a new indication for its anti-cancer drug, Gazyva (obinutuzumab), in treating follicular lymphoma.

The US Patent and Trademark Office issued three new patents that extend protection for Alexion’s rare-disease drug, Soliris, for an additional 10 years.

Lonza further expands its micronization services with the acquisition of Swiss contract manufacturer, Micro-Macinazione, following its previous $5.5-billion acquisition of dosage form provider, Capsugel.

Recipharm set up a manufacturing line at its facility in Sweden using LIDD’s NanoZolid technology.

Catalent Applied Drug Delivery Institute announced a partnership with Rutgers University to examine the challenges of pediatric drug formulation and delivery.