
Almac's Clinical Services business unit has expanded its service offering for dispensing and bottling solid dosage products.

Almac's Clinical Services business unit has expanded its service offering for dispensing and bottling solid dosage products.
A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services

Catalent Pharma Solutions has acquired a license to market Redwood Bioscience 's proprietary SMARTag precision protein-chemical engineering technology.

Astellas and Ambrx have entered into a collaboration to discover and develop novel antibody drug conjugates (ADCs) for an undisclosed number of targets in oncology. ADCs enable targeted delivery of drugs to the target tissue.

Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.
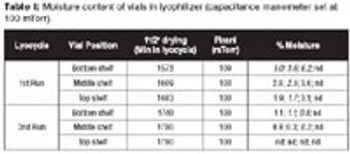
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

This article discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.
Industry experts discuss the benefits and challenges of self-administration of injectable therapies.
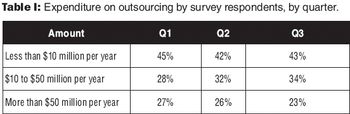
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.

Vetter held a groundbreaking ceremony for its new facility in Ravensburg.
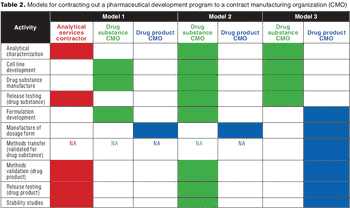
Formulation strategy is an important consideration when selecting and managing outsourced biopharmaceutical development programs.
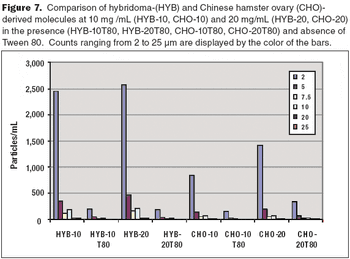
How to maintain product stability and prevent particulates.
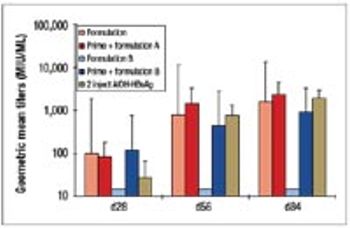
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.
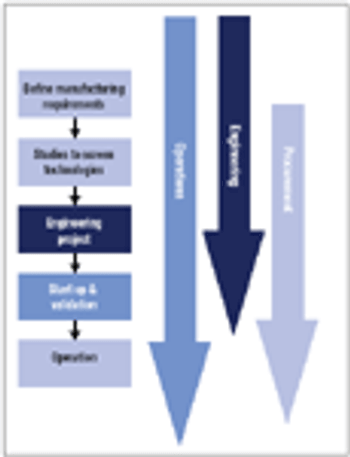
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.
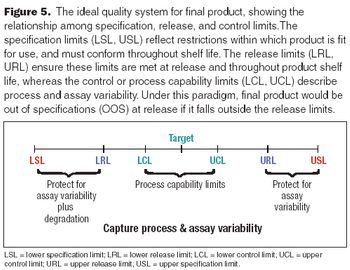
Set limits to provide incentives for process improvements.
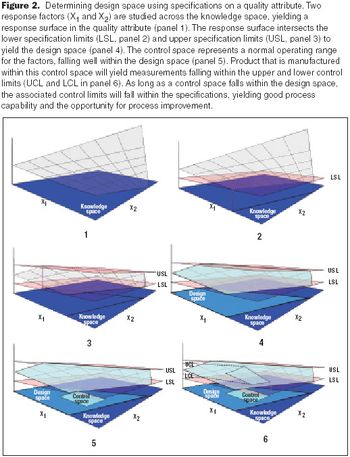
Resolve confusion about measurements.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.
Nothing beats a good dictionary. It can clarify doubts, settle an argument, or prompt exploration into new areas of learning.

The transdermal delivery of biologics-as well as of conventional drugs-is growing in popularity because the technique offers numerous advantages.