
Primary packaging and container design reflects a move to patient-friendly formulations and delivery systems.

Primary packaging and container design reflects a move to patient-friendly formulations and delivery systems.

A Q&A with PCI Pharma Services about best practices for choosing and maintaining temperature sensors in cold and cryogenic storage.

GS1 US published new guidelines in preparation for the serialization and traceability requirements associated with DSCSA.

UPS entered into a definitive purchase agreement to acquire Marken, a provider of supply-chain solutions for the life-science industry.

Vendor selection and materials testing are complex enough, but in today’s volatile environment, risk mapping and monitoring are also crucial.

TraceLink launched the EU-hosted early access program to reduce the time, cost, and risk of achieving regulatory compliance for global requirements.

GENCO, a FedEx Company, introduces a multi-tenant warehouse model in Milton, Ontario, and Memphis, Tennessee.

A technology management process identifies and evaluates new technologies in biopharmaceutical manufacturing to aid business decisions.
As the pressure to bring drugs to market more quickly increases, companies are faced with the challenge of selecting the most effective cold-chain storage solutions.

Onset expanded its product line for pharmaceutical cold-chain management with the launch two new products.

Researchers from the Wyss Institute explain a potential method for transporting and producing temperature-sensitive pharmaceuticals at a reduced cost.

Airlines, airports, freight forwarders, and other cold-chain partners are taking a crash course in pharma cGMPs.

CSafe completed the acquisition of Kallibox, a manufacturer of cold-chain packaging solutions.

The company is opening two offices in the United States that will offer serialization, automation, and process control services.

The company opened a new service center in San Juan, Puerto Rico.

Zenith appointed Carlos Machado as serialization director to lead the company’s sales, operations, and post project support services.

Recent trends in raw materials packaging may impact manufacturing, quality, and cost of biopharmaceuticals.

Planning ahead is key to enabling a continuous and secure supply chain that adapts to changes in market demand.

Progress is being made, but work remains to be done. Brian Daleiden, vice president of marketing at TraceLink, discusses the state of the industry, as companies attempt to meet complex, and changing, global requirements.

Risk management takes center stage, and new mobile apps are being developed to help assess global risks in real time.

Real-time GPS technology, better IT connections, and more conservative, controlled shipping temperatures are improving the shipment of sensitive pharmaceuticals.
The authors provide their perspectives on shipping validation.

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.
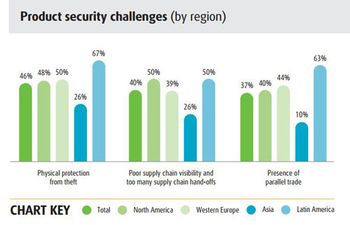
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

The new IVIG products boast improvements in purity, safety, and yield.