
The revised guidance aims to strengthen the agency’s global approach to develop new antibacterial medicines.

The revised guidance aims to strengthen the agency’s global approach to develop new antibacterial medicines.

Orphan and cancer drugs continue to lead, but treatments for many common diseases were also approved in 2018.

The plan details five goals to guide the development of Sentinel, a national electronic system for medical product safety surveillance established as part of the FDA Amendments Act of 2007, over the next five years.

The company is recalling 5 lots of Ceftriaxone for Injection, USP, 250mg; 10 lots of Ceftriaxone for Injection, USP, 500mg; 24 lots of Ceftriaxone for Injection, USP, 1g; and 3 lots of Ceftriaxone for Injection, USP 2g because of the presence of particulate matter in reconstituted vials.

In 2018, the agency authorized 42 drugs with new active substances, three advanced therapies, and 21 orphan drugs.

FDA sent a warning letter to Skylark CMC Pvt. Ltd. after employees refused to let agency inspectors enter the company’s Ahmedabad, Gujarat, India facility.

Pricing pressures, investment volatility, and government disfunction greet Biopharma in 2019.

Bio/pharma companies are successfully launching novel therapies; however, the industry still needs to work on manufacturing innovation.

FDA plans to support initiatives to ensure that all medicines are safe, effective, and of high quality.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

Core functions and those funded by fiscal year 2018 user fees are continuing, and 59% of the agency’s staffers are being retained.

The agency sent a warning letter to Roche’s Genetech for marketing unapproved stem cell products and puts other stem cell firms and providers on notice.

Asclemed USA Inc., dba Enovachem Pharmaceuticals is recalling the product due to labeling that incorrectly states that stoppers do not contain latex.

The agency sent a warning letter to Cao Medical Equipment Co., Ltd. after inspectors found CGMP violations at the company’s Langfang, Hebei facility.

FDA has withdrawn the proposed rule that would have allowed generic-drug makers to independently update and distribute new safety information in drug labels.

FDA cites Zhejiang Huahai Pharmaceutical in valsartan impurity investigation.

New FDA guidance developed to identify lapses in data integrity and promote best practices.

The agency is looking to leverage real-world health data to support drug development.

FDA has approved Truxima (rituximab-abbs), a biosimilar to Roche’s anti-cancer biologic, Rituxan (rituximab).

Shire has announced that the European Commission has granted marketing authorisation for Takhzyro (lanadelumab) subcutaneous injection.

The proposal from the Centers for Medicare and Medicaid Services (CMS) proposes to give plans more flexibility to limit coverage of certain drugs.
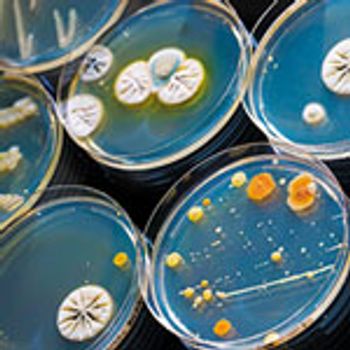
Microbial identity data can be critical for determining contamination sources.
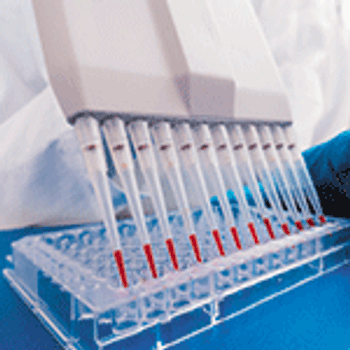
Unprocessed bulk material harvested directly from the bioreactor should be tested for contamination prior to downstream processing.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business.