
Efforts by regulators seek common approaches to clinical research and biopharmaceutical production needed to wipe out the coronavirus pandemic globally.

Efforts by regulators seek common approaches to clinical research and biopharmaceutical production needed to wipe out the coronavirus pandemic globally.

Time sure flies, except when you are waiting for something to happen.

When in-person training may not be feasible, there are still opportunities to ensure employees receive the required training, says Susan J. Schniepp, distinguished fellow, Regulatory Compliance Associates.

Glycoengineering is growing in importance as a technique to improve antibody therapeutic efficacy, safety, and product quality.

Patient advocates, research experts, and industry are calling for more user fee revenues to support CBER programs to advance innovative therapies.

Should therapies and vaccines be cheap or free in a pandemic and would that really dry up innovation?

Industry should be seeking more information from FDA on how it will restart its current inspection program.

The agency is hoping to restart performing on-site domestic inspections during the week of July 20, 2020 depending on factors such as the status of COVID-19 in the state of inspection and local rules and guidelines.

FDA officials are moving to clarify standards and requirements for vetting and approving viable preventives.

Innovative approaches, including ready-to-use materials and in-line dilution, can significantly streamline overall bioprocessing operations.

Identifying the source, assessing the risk, and removing residual impurities requires a strategic approach.

Investigating the root causes of OOS conditions, product defects, and batch failures requires a systematic, thorough approach.

Determining the root cause of out-of-specification (OOS) and other problems requires a careful, deductive approach and a clear understanding of processes.
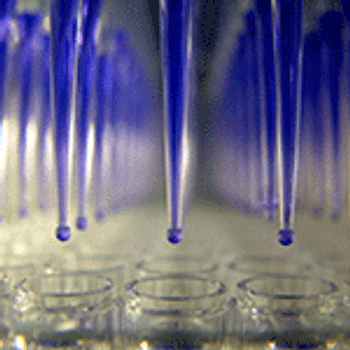
Appropriate analytical assays are needed to determine and ensure that biosimilar critical quality parameters are on track.

Safeguarding against microbial contamination requires rapid detection and innovative technology.

The right approach to biological safety cabinets, and collaboration between engineers and those who will operate the equipment, is crucial to preventing cell-culture contamination.

Data and science must guide FDA in making pressure-filled COVID-19 vaccine and therapy approval decisions.

FDA can better monitor quality production of domestic versus foreign firms.

It is important to consider the feasibility, benefits, and limitations of each type of audit in advance.

Phesgo is a fixed-dose-combination subcutaneous injection of Perjeta (pertuzumab) and Herceptin (trastuzumab) for treating HER2-positive breast cancer.

The complete response letter was issued for a biologics license application for Abicipar pegol, an investigational treatment for wet age-related macular degeneration, based on FDA’s determination of an unfavorable benefit–risk ratio.

The guidance document provides recommendations regarding data needed for the manufacturing, development, and approval of a COVID-19 vaccine.

The company now offers its CONFIDENCE virus clearance services to support validation of viral clearance processes.

FDA is placing emphasis on developing guidance documents more quickly and efficiently, and agency officials expect that such approaches may continue in the future.

Members of the European Commission, EMA, and FDA met in a virtual two-day meeting to discuss ongoing joint initiatives and upcoming priorities.