
The authors present a risk analysis of the impact of various business and operating risks on three facility layout strategies.

The authors present a risk analysis of the impact of various business and operating risks on three facility layout strategies.
This article explores the challenges and potential of next-generation therapeutic antibodies.

CDMO, Vibalogics, has revealed that it will be acquired by a private equity firm, Ampersand Capital Partners.

A new facility to be built in the Philadelphia Navy Yard will support commercial production for autologous tumor-infiltrating lymphocyte cell therapy products from Iovance Biotherapeutics.

By connecting manufacturing processes and logistics technology, GE Healthcare and World Courier look to accelerate the development of advanced therapies.

The expansion will increase the company’s single-use capacity to meet growing demand.

Catalent expands gene therapy capabilities with $1.2-billion acquisition of Paragon Bioservices.

The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

The acquisition of GlaxoSmithKline’s manufacturing site in Cork, Ireland, will expand Thermo Fisher Scientific’s global footprint for complex API manufacturing.

The partnership will focus on the commercialization of inducible promoters to improve biomanufacturing.

GE Healthcare launches Chronicle GMP-compliant automation software for cell therapy manufacturing.

The new 40,000-ft2 facility will support Bayer's growing biologics portfolio in oncology, cardiology, and additional therapeutic areas.

Harvard University researchers used single-cell sequencing to identify a protein expressed uniquely by insulin-producing beta cells created from stem cells in the laboratory.

Vibalogics has increased its single-use bioreactor and purification capacity with a new manufacturing for its specialist oncolytic virus and viral vector manufacturing services as a result of a growth in demand.

Dyadic International has announced its collaboration agreement with Serum Institute of India for the development and manufacture of up to 12 antibodies and vaccines using Dyadic’s C1 gene expression system.

It has been confirmed that both Bulgaria and Cyprus will now be able to perform GMP inspections at a level considered equivalent to the United States

AbCellera, Niaid Vaccine Research Center, and Ichor Medical Systems have formed a partnership to develop an end-to-end platform capable of developing field-ready, nucleic acid-based countermeasures against a pandemic strain of influenza.

A solar panel installation at Novo Nordisk’s North Carolina facility was initiated in March 2019 as part of the company’s commitment to zero environmental impact globally.

MilliporeSigma event scheduled to recognize biotech challenges and support development potential.

The Vaccine Development and Bioprocess Cell Culture Technology Day will take place on May 16, 2019 in Baltimore, MD.

In collaboration with Pall Corportion, biotech company Freeline completed the first full-scale run at its newly commissioned, GMP gene-therapy manufacturing facility in the UK.
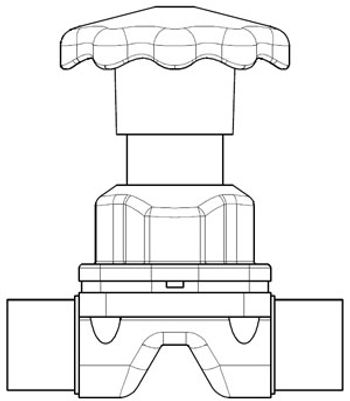
Valve design and materials affect performance and cost to maintain.

Recent upstream processing innovations include enhanced sensor technology, single-use bioreactors, and automated cell culture systems.

This article presents some key differences between the US and European regulation of biosimilars, including naming conventions and pharmacovigilance of biosimilars, and the impact of biosimilars on commercialization and affordability of biotherapeutics.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.