
Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Industry experts discuss significant achievements. Plus: What's in store for the future.

Ray O'Connor, an operations consultant with NIBRT, addresses aseptic processing, including how to avoid contamination, and cleanroom best practices. Posted May 2012.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has formally adopted a memorandum of understanding (MoU) with key partners for a harmonised, European system for medicines verification.

The UK's Medicines and Healthcare products Regulatory Agency has launched a new anticounterfeiting strategy with the aim of curbing the occurrence of falsified medicines in the county's supply chain.
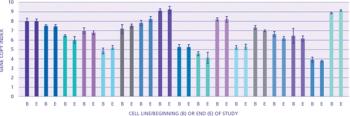
The author describes expression technology that produces cell lines with high genetic stability.

No matter the time or cost, knowing what's going on inside your facilities is always going to be worth the effort.
Industry experts discuss the benefits and challenges of self-administration of injectable therapies.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.
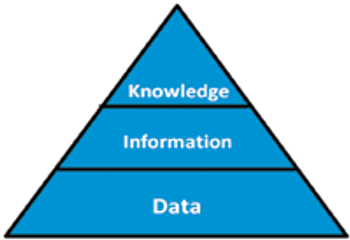
The authors discuss the technology and guidance required to achieve good KM in a biopharmaceutical company.

The authors describe a new assembly for bulk and final drug product filling operations.
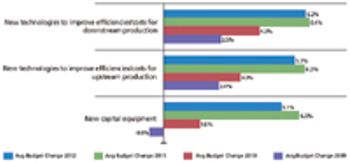
New outsourcing industry sectors have become multibillion-dollar industries.

Challenges of vaccine development include regulatory, technical, and manufacturing hurdles in translating a vaccine candidate into a commercial product.
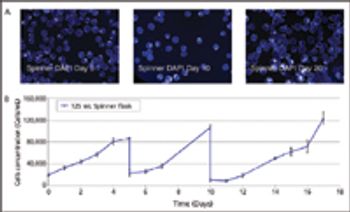
Demonstration of large-scale stem-cell scale-up.
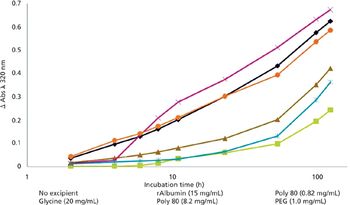
Recombinant albumin can stabilize a drug product and assist in API release.

Key business considerations when developing biosimilar products virtually.

When a pharmaceutical supply chain is compromised, there can be disastrous consequences, not only for consumers, but also for manufacturers. Without comprehensive security measures, pharmaceuticals are susceptible to counterfeit, diversion, dilution, tampering and deliberate contamination ultimately compromising patient safety.
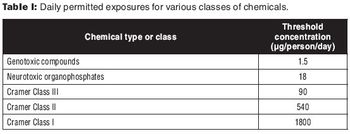
This article is the second in a two-part series on extractables and leachables.

This article outlines methods, validation standards, and documentation of sterilization of single-use products using gamma irradiation.

This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."

The authors describe a novel means to control ice nucleation using a sterile cryogenic ice fog.

Virtual biotech CEO Anjan Selz focuses on product development, credibility and differentiation.

The author describes automated equipment that uses functionally closed disposables.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

The EMA released a concept paper for consultation on Nov. 8, 2011, that recommends a revision to Annex 16 of the Guide to Good Manufacturing of Medicinal Products to address more complicated global supply chains and new falsified medicines legislation. Since Annex 16 was introduced in 2001, a number of positive and negative trends have occurred in the pharmaceutical industry. In particular, confusion has arisen over the role of the qualified person (QP), and harmonization of requirements has been lost between member states. The EMA cites several commonly asked questions within the concept paper: