
The author provides a review of the concepts of design and qualification that apply to single-use systems.
Jerold Martin was the senior vice president of global scientific affairs at Pall Life Sciences and chairman of the Board and Technology Committee at Bio-Process Systems Alliance.

The author provides a review of the concepts of design and qualification that apply to single-use systems.

BioPharm International eBooksVolume 28, Issue 14 Single-use technologies including polymer film bags, tubing, filter capsules, and appropriate connectors have become widely accepted in bioprocessing of proteins and vaccines.

New publications from BPSA and PDA highlight best practices for particulate control, quality agreements, and implementation strategies.

Progress is being made in the development of harmonized best practices for single-use systems.

Studies show diminished cell growth properties associated with some biocontainer or bioreactor films.

Choosing the right disposable components for your application.

Jerold Martin considers the types of tubing available to the industry and how to make an informed selection.
This month, Jerold Martin of Pall Life Sciences takes a look at protein recovery through direct-flow microporous membrane filters over the past 25 years.

The author considers the types of tubing available to the industry and how to make an informed selection.

There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

This article outlines methods, validation standards, and documentation of sterilization of single-use products using gamma irradiation.

Single-use manufacturing may seem like a new trend, but it has actually been around for almost 30 years, beginning in the early 1980s when filter manufacturers began to make small process-scale plastic filter capsules to replace "junior" size stainless-filter housing assemblies.

In this quarter's column, highlights from the IBC Single-use Applications meeting, the PDA Single-use Workshop, and the BioProcess systems Alliance International Single-Use Summit are presented.

Developing a quality agreement template for single-use systems.

The author looks at strategies to minimize particle levels in the finished product when using single-use technologies downstream of final capabilities.

Single-use conference roundup.

Although some aspects of single-use components can be standardized, it is unlikely that any materials or design features will become a commodity

BPSA eases confusion over extractables and leachables testing through guides.
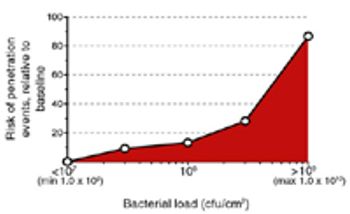
Filterability and bacterial retention must be verified very early in process development to ensure successful sterilizing filtration validation.
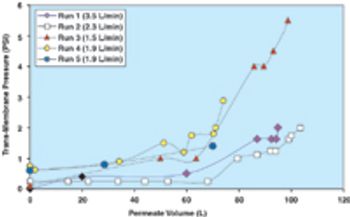
Filtration is one of the most commonly used unit operations in the manufacturing of biopharmaceuticals. This is the second part of the fourth article in the "Elements of Biopharmaceutical Production" series. In this second segment, Manoj Menon and Frank Riske present an approach for the development and optimization of a TFF application, followed by a contribution from Jennifer Campbell and Elizabeth Goodrich reviewing key issues involved in validation of a TFF step.
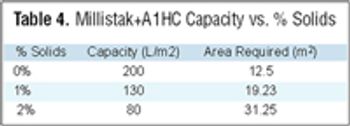
Filtration is one of the most commonly used unit operations in biopharmaceutical manufacturing. Available formats include direct or normal flow filtration (NFF) and cross or tangential flow filtration (TFF). These methods are used for sterilization and virus filtration, depth filtration or ultrafiltration, and diafiltration applications. Some common objectives include:

Biopharmaceutical companies can access their suppliers? resources to reduce validation and compliance efforts.

Published: February 1st 2014 | Updated:

Published: August 1st 2013 | Updated:

Published: April 2nd 2013 | Updated:
Published: November 1st 2012 | Updated:

Published: November 1st 2012 | Updated:

Published: November 1st 2011 | Updated: