Choosing the right tools to enhance the process.

Choosing the right tools to enhance the process.
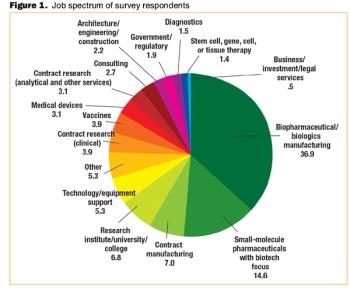
Will the global economic crisis affect your job? BioPharm International's third annual salary survey finds out.

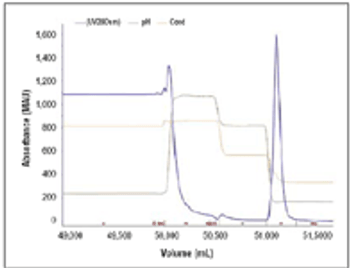
Results from a process developed for a commercial antibody.
FDA perspectives on specs and effective control strategies.
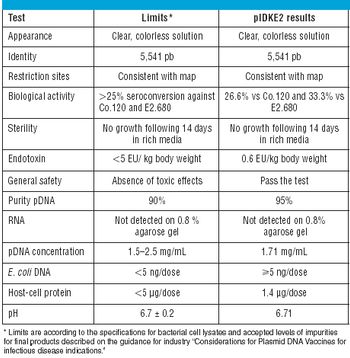
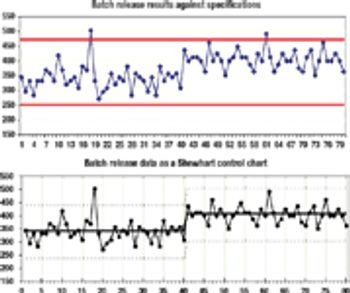
Improving your quality operations by using sound statistical principles.
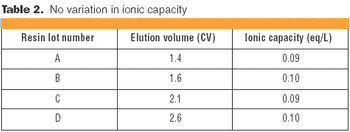
Understanding the impact on process performance.

In the context of process validation, the confirmation of a belief must be checked repeatedly, throughout the product lifecycle.
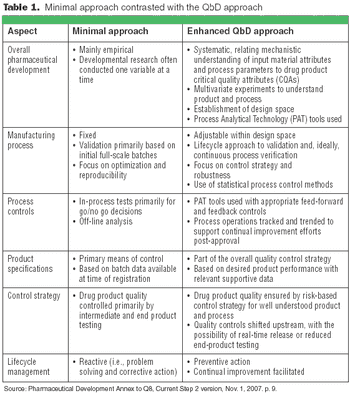
The principles of QbD can be applied to biotech development and manufacturing to help resolve many common issues. QbD scientifically provides a greater understanding of the complex relationships among product quality attributes, the manufacturing process, and clinical safety and efficacy by determining the various permutations of critical input variables that will keep the product within specification.
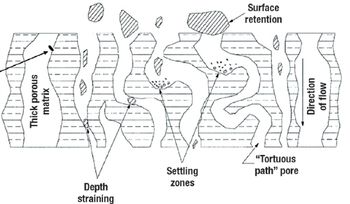
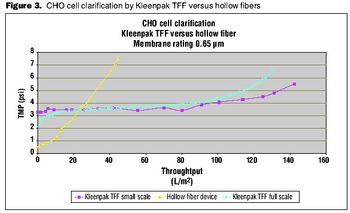
A comparison of primary harvest techniques.
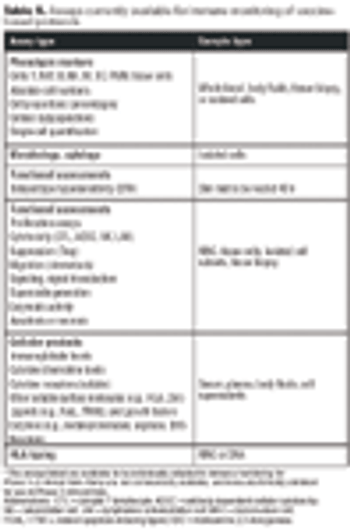
Emerging therapies pose challenges for standardizing QC.
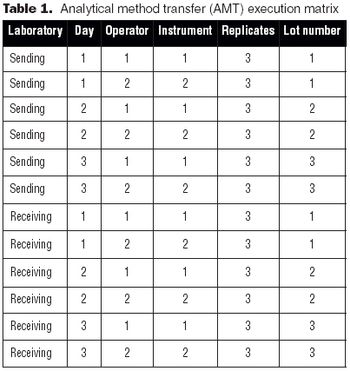
It is essential to understand the critical elements of validation extensions to ensure accurate process or product quality measurements.
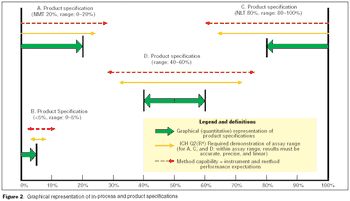
Validation of analytical methods can be more easily accomplished by breaking the task down into a series of planned steps.
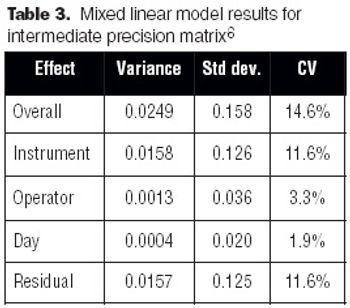
The development and optimization process can improve a method, but validation does not. Validation is the final proof that regulations and expectations are met.
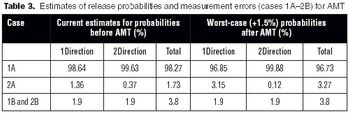
Several steps can be taken to maintain test method suitability after the formal completion of the analytical method validation studies,

Two case studies illustrate a systematic approach.

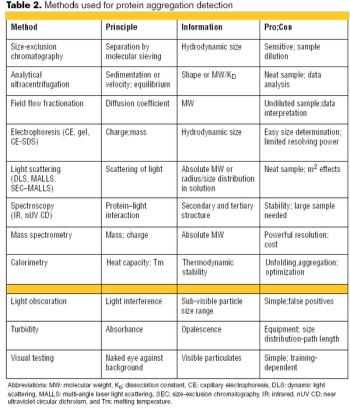
Both innovator and generics companies are using analytics to support comparability arguments.
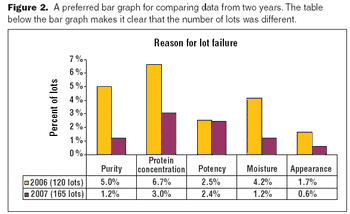
The key to a good graphical presentation is to select the method that best fits the data.

Design space concepts are key to a successful technology transfer.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.

Contrary to popular belief, the out-of-specification problem started years before the Barr Decision.

Regulatory agencies have evolved along with the biotechnology industry to define quality standards.

Multivariate data analysis can help biotech manufacturers deepen their process understanding.
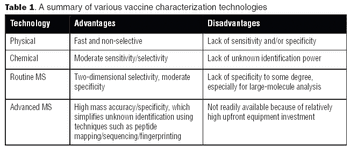
With the advent of high-resolution mass spectrometers and highly sensitive MS instruments, vaccine characterization has entered a new phase.