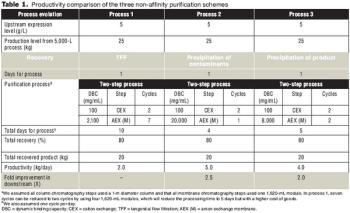
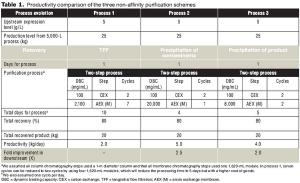
In three non-affinity purification processes based on cation exchange capture with high binding capacity, applying a host cell protein exclusion strategy enabled robust scale up and better economics.
Gisela Ferreira, PhD, is a senior process engineer, purification process development, at Medarex Inc.

In three non-affinity purification processes based on cation exchange capture with high binding capacity, applying a host cell protein exclusion strategy enabled robust scale up and better economics.
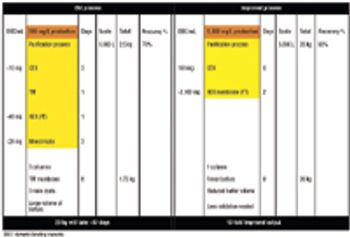
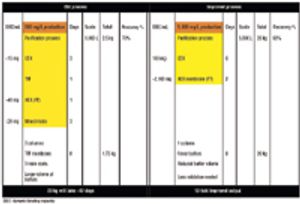
Affinity purification schemes for antibody production have certain limitations keeping up with cell culture expression levels as they reach and exceed 10 g/L. New downstream purification processes are based on low cost, long lasting, and high binding (40–100 mg/mL) cation exchange resins.
Published: March 2nd 2009 | Updated:
Published: February 2nd 2007 | Updated: