
A Q&A on bioanalytical method integrity with Roger Hayes of MPI Research.

A Q&A on bioanalytical method integrity with Roger Hayes of MPI Research.

Methods must be suitable at each development phase, robust, and effective on multiple platforms.

Development and validation of two pivotal assays for in-vitro comparability testing of insulin biosimilars.

An automated analytical method determines the purity of chromogenic, fluorescently tagged proteins or metal-bound proteins.

Industry players form Allotrope Foundation to solve analytical data management problems.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss special considerations for testing for extractables and leachables for both small molecule and biological drug development.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss the key elements of an effective testing program to identify extractables from materials and leachables in drug products.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss new analytical testing procedures and instruments that are facilitating the testing processes or improving results.

Editorial Director Rita Peters talks to with Ferdinand Dabu Director of Marketing at SGS live at Interphex
The author describes a method to avoid protein aggregation when using light scattering systems.

As biopharmaceutical/pharmaceutical companies increase their development of biologic-based drugs, companies providing analytical instrumentation and laboratory testing goods and services are, in turn, offering improved tools for biologic characterization, biomanufacturing, and related testing.
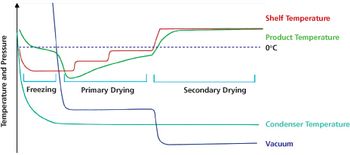
Optimized freeze-drying cycles can offer scientific and business advantages.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.
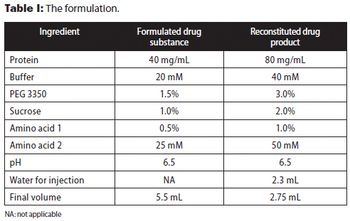
The authors present approaches used to reduce reconstitution time of a lyophilized high-concentration protein drug product.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

NIBRT's Michael Lacey provides an overview of biopharmaceutical facility design and operation.

Project: transfer a manual concentration/diafiltration process for siRNA production.

FDA talks about the changing scope of regulatory science.

What the industry's future holds and what needs to be done to get there.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

Preparation of biological samples for chromatographic analyses.

NIBRT's Pauline Rudd on what to expect when performing glycan analysis.

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?

NIBRT's Jayne Telford provides an overview of biopharmaceutical analytics and their accompanying qualification and validation steps.

A 10-step systematic approach to analytical method development and validation can improve the quality of drug development.