
These articles encapsulate the past, present, and possible future of process-scale chromatography in biopharmaceutical production.

These articles encapsulate the past, present, and possible future of process-scale chromatography in biopharmaceutical production.
This article discusses how on-line high-performance liquid chromatography (HPLC) can measure product purity in the column eluent stream in near–real time. These data can then enable the automation and control of a purification column operation, thus reducing product variability, shortening process cycle time, and increasing yield. An example application demonstrates how on-line HPLC is used as a process analytical technology to ensure the process can accommodate variability in the separation while ensuring the product meets its critical quality attributes.
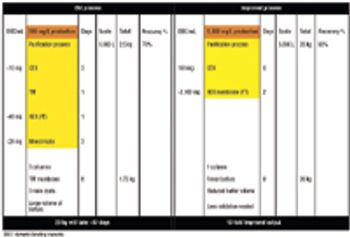
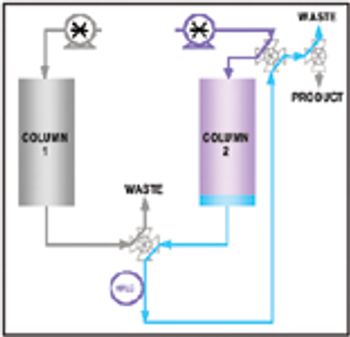
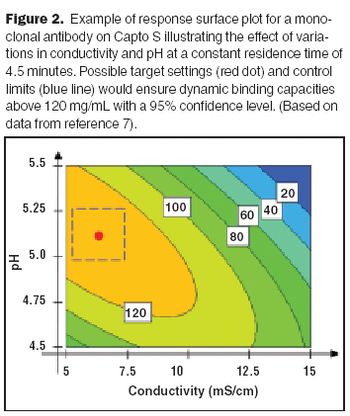
Affinity purification schemes for antibody production have certain limitations keeping up with cell culture expression levels as they reach and exceed 10 g/L. New downstream purification processes are based on low cost, long lasting, and high binding (40–100 mg/mL) cation exchange resins.

Vagueness in the ICH Q2A and Q2B guidelines necessitates effective protocol design and data analysis. For specificity (detection in the presence of interfering substances), the goal is statistical differences with meaningful implications on assay performance. Linearity (results directly proportional to concentration of analyte in the sample) is typically demonstrated via least squares regression. Accuracy (difference between measured and true values) usually is presented as a percent of nominal. Precision analysis is vital because it supports claims of accuracy and linearity. A well-designed experiment and statistically relevant methods will facilitate method validation in accordance with ICH guidelines.

Increased resin stability can extend the number of cleaning cycles that can be performed in situ.

This article shows how Probabilistic Tolerance Intervals of the form, "We are 99% confident that 99% of the measurements will fall within the calculated tolerance limits" can be used to set acceptance limits using production data that are approximately Normally distributed. If the production measurements are concentrations of residual compounds that are present in very low concentrations, it may be appropriate to set acceptance limits by fitting a Poisson or an Exponential Distribution.

The first part of this article, published in the September 2006 issue, discussed general strategies for validation extensions to other test method components, laboratories and even different test methods.1This second part provides practical tips on how to maintain test method suitability long after the formal completion of analytical method validation (AMV) studies.

Good risk management tools dictate how much assay performance characteristics can deviate from ideal.
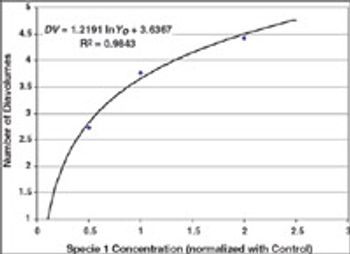
Case studies were run to test Process Analytical Technology applications for protein refolding, diafiltration, and cation exchange chromatography. It is shown that it is feasible to design control schemes that rely on measurement of product quality attributes and thereby enable real-time decisions.

A novel calibration approach was developed that not only calibrates the X-axis, but also calibrates the peak shape.

For decades now, it has been said that "the process is the product" for biologics. Great care and consistency must be applied in their upstream manufacture-during fermentation, harvest, and early purification-to preserve their complex structure, which confers their activity and specificity. As the product moves to late-stage purification, however, the relative concentration of impurities and altered product forms is diminished. Also, the final dosage form of most large molecule biopharmaceuticals is the relatively simple liquid formulation of parenteral dosage form. In contrast, manufacturing the solid dosage forms common for small-molecule drugs involves more complex processes, such as mixing dry powders, granulation, manufacturing controlled-release matrices, and tableting.

"Clinical data is the gold standard" for setting manufacturing specifications, said Patrick Swann, PhD, acting deputy director of the Division of Monoclonal Antibodies at FDA, at a session on specification setting at the AAPS National Biotechnology Conference that was held June 19-21 in Boston.

Protein aggregation is a term that can include many types of aggregation, from rapidly reversible aggregation caused by non-covalent bonds to irreversible aggregation in the form of covalent oligomers.

Using packed columns in process development activities limits the scope for appraising a large and diverse range of media.

Signal-to-noise ratios are useful in robust engineering to design products and processes that consistently deliver on target.

Extra column effects must be accounted for to make a valid comparison

Lyophilized, or freeze-dried, materials are challenging samples for quality assurance and quality control (QA/QC) measurement because of the inability to open the container without corrupting the product. Near-infrared analysis presents itself as the QC method of choice for lyophilized materials due to its ability to penetrate glass or plastic containers to analyze the sample in a non-destructive manner. This study demonstrates the performance of a Fourier transform near-infrared (FT-NIR) spectrometer used in analyzing lyophilized samples of thrombin, a topical coagulant commonly used in the medical and dental fields. Key stability parameters for lyophilized thrombin include moisture and potency, which can be predicted simultaneously from a single spectrum using multivariate analysis.
Ultra performance liquid chromatography (UPLC) is a new category of liquid chromatography that researchers are using to increase resolution, speed, and sensitivity in a variety of applications. These benefits result from packing columns with 1.7 ?m particles and using instruments that are optimized for such columns.
PAT can be defined as a collection of real-time data in-line to make decisions about product quality early in the production process.

Many industry professionals know that analytical testing for biopharmaceuticals for all raw materials, production in-process stages, and final containers must be validated, and they generally understand how this can be achieved. Many of us even understand the basic concepts of laboratory compliance and production process quality. However, how exactly are analytical test method performance and process robustness related and how do they depend on each other? Furthermore, how do we monitor and maintain the accuracy and reliability of analytical methods long after validation completion to ensure the suitability of these methods for measuring process quality?

Departure from dilutional similarity can be interpreted as evidence that the groups of organisms are not comparable or the preparations do not contain the same active compounds.

FDA and regulatory agencies worldwide have recently approved many advanced bioanalytical technologies. Receiving approval of advanced test methods for new biopharmaceutical products is relatively straightforward, provided clinical and process validation data are generated by the same (or at least similar) test method. However, regulatory approval becomes more difficult and time consuming when compendial test methods or test methods for already licensed biopharmaceuticals are changed.

Characterization of biopharmaceuticals (proteins) during early development is done for several reasons. The most important reason is the need to have supporting data that demonstrates the comparability of material used throughout development. This is particularly important as the production process is optimized and small changes in the process may affect the structure of the product. Demonstration of comparability of proteins produced throughout product development is more complicated, due to the inherently heterogeneous nature of many biologicals.

The methods used in most microbiological test laboratories originated in the laboratories of Koch, Lister, and Pasteur. While numerous changes have occurred in the chemistry laboratory, there have been limited improvements in methods used for microbiological testing.