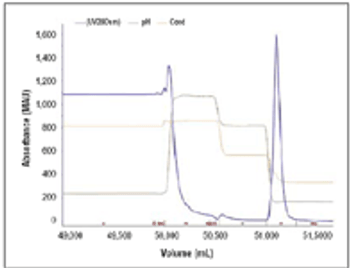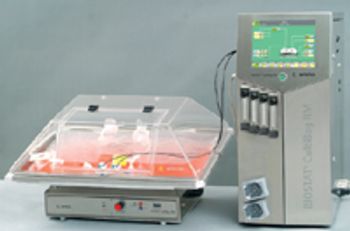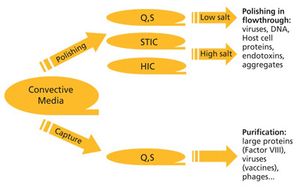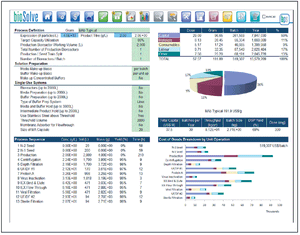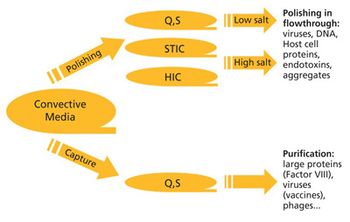
As constant scale up grows out of favor in the biopharmaceutical industry, new-and old-approaches are required. The author reviews the state of downstream processing and considers potential solutions, including the streamlining of full processes and borrowed technologies