
IDBS described benefits and best practices for laboratory electronic data systems.

IDBS described benefits and best practices for laboratory electronic data systems.
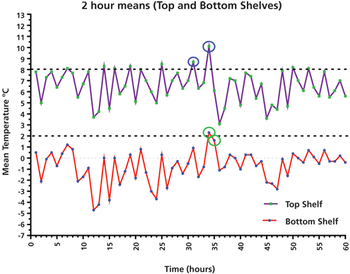
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.

Method choice is crucial to when seeking answers to biosimilar characterization questions.

New reports address biopharma’s leading concerns: funding for drug development and pricing of finished drugs.

The opening presentation gives the company a chance to put their best foot forward, according to Siegfried Schmitt, principal at PAREXEL.

This article presents a general strategy for authorship of deviation investigations, with primary focus on regulatory inspection success.

The authors summarize the current regulatory expectations regarding the number of PPQ batches required and provide potential approaches that can be used to determine and justify the number of PPQ batches.
While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

Single-use bags containing toxic or hazardous materials required special handling.

CDER’s Janet Woodcock endorses modern drug manufacturing to ensure access to safe and reliable medicines.

How has the bio/pharmaceutical contract manufacturing industry evolved and changed over the years and what does the future hold?
The authors present a shift toward more integrated purification processes.

Perfusion processes can attractive for biologics drug manufacturing; however, obstacles remain.

Continuous processing of 100 g of monoclonal antibody in 24 hours has been demonstrated using lab-scale equipment.

Electronic systems can remove opportunities for individuals to make mistakes or to manipulate the data.

Optimize practices and meet requirements using electronic data integrity systems.
Short tandem repeat profiling is the most validated method to confirm cell line identity and avoid misidentified or cross-contaminated cell lines.

Click the title above to open the BioPharm International July 2017 issue in an interactive PDF format.