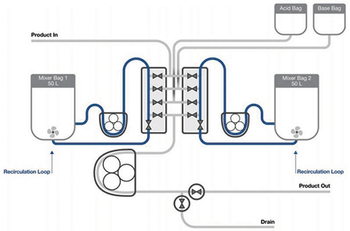
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.
Mark Schofield, PhD, is senior R&D manager, Pall Life Sciences.

Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

Perfusion processes can attractive for biologics drug manufacturing; however, obstacles remain.

Continuous processing of 100 g of monoclonal antibody in 24 hours has been demonstrated using lab-scale equipment.

Published: July 1st 2017 | Updated:

Published: July 1st 2017 | Updated:
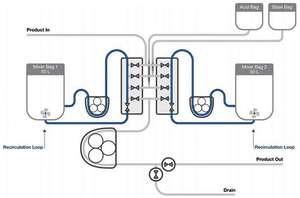
Published: September 28th 2018 | Updated: