
The agency will employ a ring vaccination method similar to the one used to eradicate smallpox.
Randi Hernandez was science editor at BioPharm International from September 2014 to May 2017.

The agency will employ a ring vaccination method similar to the one used to eradicate smallpox.

FDA approved Sandoz’s Zarxio (filgrastim-sndz) on March 6, 2015. The approval is a groundbreaking decision, as Sandoz is the first pharmaceutical company to have a biosimilar product approved in the United States. Known as Zarzio outside of the US, Sandoz says its biosimilar filgrastim is already available in more than 60 countries worldwide, has generated more than 7.5 million patient-days of exposure, and is "the most widely used filgrastim in Europe."
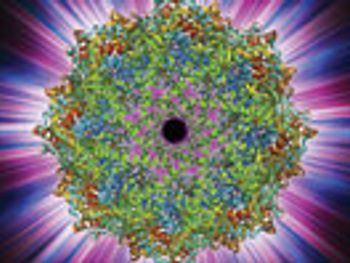
The thorough analysis of a therapeutic protein product’s propensity to aggregate may be a necessary step in the prevention of a cell-mediated immune response.

Remsima will now be available for patients in 12 additional countries in the European Union.

Brandicourt will leave Bayer HealthCare AG to begin his new role as CEO of Sanofi in April 2015.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

The draft guidance contains policies for drugs that are processed with additional manufacturing steps such as remixing, dilution, and repackaging.

Actavis says it plans to use the Allergan corporate name for its branded products.

Hospira’s Inflectra (infliximab), a biosimilar for Remicade, is approved by the European Commission.

Antibodies in research should be standardized and categorized using a barcode-like classification system, according to research published in Nature.

The new center represents AmerisourceBergen’s first facility erected specifically for clinical trial and commercial third-party logistics activities.

Genzyme will partner with Voyager Therapeutics for the discovery, development, and commercialization of novel gene therapies for central nervous system disorders.

On February 3, 2015, the FDA published a notice in the Federal Register that it is soliciting input on the collection of data to support interchangeability claims in biosimilar applications.

The recent mergers, partnerships, and incentive-laden deals in pharma may keep the industry from continuing to experience diminishing returns, according to IMS’ Michael Kleinrock.

The deal may offset billions of dollars in waning sales from Pfizer drugs slated to lose patent protection and provides Pfizer with a whole portfolio of biosimilar products.

The cyclin-dependent kinase 4/6 inhibitor for the treatment of metastatic breast cancer was approved more than two months ahead of the prescription drug user fee goal date.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.

EMD Millipore will provide process development services for Precision Biologics’ preclinical monoclonal antibody.

FDA says it is “weighing the appropriate regulatory approach” to handle the tasks outlined by President Obama’s new Precision Medicine Initiative.

Developing intellectual property standards for biological products is a point of conflict as negotiations on the Trans-Pacific Partnership continue.

AstraZeneca will team up with various organizations to employ CRISPR technology for precise gene editing in recombinant cell lines.

The pharmaceutical manufacturer pledged to freeze vaccine prices for Gavi-eligible countries for a decade.

The potential blockbuster treatment targets a protein involved in cholesterol homeostasis.

Following a proposal by the World Health Organization, Australia will abandon a previously proposed update in biosimilar nomenclature.

Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

A new study conducted by the National Institutes of Health found that a certain vector used in gene therapy (and its insertion site in the genome) may be associated with an increased risk of liver cancer.

Cosentyx (secukinumab) is the first IL-17A inhibitor for moderate-to-severe plaque psoriasis patients.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

The move represents Hospira’s first biosimilar submission in the United States.