
FDA sent a warning letter to Lernapharm (Loris) Inc. detailing the company’s lack of procedures to prevent microbiological contamination.

FDA sent a warning letter to Lernapharm (Loris) Inc. detailing the company’s lack of procedures to prevent microbiological contamination.

FDA is revising its inspection process and seeks harmonization of standards for US and foreign regulatory oversight to ensure the safety of medicines.

FDA, innovator companies, and biosimilar developers maneuver over exclusivity, naming, interchangeability, and more.

More than 120 healthcare organizations plan to bring competition to generic drug market.

More consistent and reliable production processes are critical for advancing innovative treatments.

Detailed process descriptions and robust documentation aid in compliance as well as training, says Siegfried Schmitt, principal consultant at PAREXEL.
This study was successful in establishing a reliable and effective method for evaluating cleaning processes based on risk. Click here to view a PDF of this article.

The US Pharmacopeial Convention (USP) is developing a new chapter for rapid sterility testing of short-life products based on the recommendations of a panel of experts and stakeholders.

The European Commission (EC) has approved GlaxoSmithKline’s (GSK) Nucala (mepolizumab) as an add-on treatment for severe refractory eosinophilic asthma in pediatric patients six to 17 years old.

PDA Technical Report 80 (TR 80): Data Integrity in Laboratory Systems is the first in a series of three technical reports PDA will publish on data integrity.

The European Commission (EC) has approved Novartis’ chimeric antigen receptor T cell (CAR-T) cell therapy Kymriah for the treatment of B-cell acute lymphoblastic leukemia and relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy.

The Senate approved a $159-million budget increase for FDA, to bring its resources up to $5.4 billion for 2019, including more than $2 billion in user fees.

FDA sent a warning letter to Kyowa Hakko Bio Co., Ltd. after inspectors found data integrity problems at the company’s Yamaguchi, Japan facility.

Guidances for regulatory changes, batch testing, and reporting address situations resulting from “no-deal” Brexit scenario.

As a contingency against border delays resulting from a “no-deal” Brexit, the Department of Health and Social Care (DHSC) directs pharma companies to stock extra medicines.

FDA approved the first generic version of EpiPen and EpiPen Jr (epinephrine) auto-injector.

FDA sent a warning letter to Apotex Research Private Limited after investigators found current good manufacturing practice violations.

FDA published a resource guide to promote responsible opioid prescribing in the treatment of animals.

BioPharm International spoke with Sharon Ayd, founder & CEO of Ayd Biopharmaceutical Consulting Services, about what the future holds when it comes to ensuring quality in biopharmaceuticals.

The agency issued a warning letter to Canadian API manufacturer, Les Produits Chimiques B.G.R, citing cGMP violations at its API facility in Pointe-Claire, Quebec.
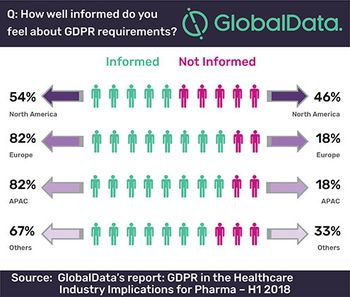
Only 54% of North Americans feel informed about the requirements of the general data protection regulation (GDPR), according to a report by GlobalData.

The type of product, the packaging materials being used, and the process and materials used to manufacture the product will determine when E&L data should be submitted to regulators, says Susan J. Schniepp, executive vice-president, Post-approval Pharmaceuticals and distinguished fellow at Regulatory Compliance Associates.

The recommended drugs include two orphan medicines and three biosimilars.

Managing data at the different stages of the lifecycle, linking disparate systems together, and making the right data available to those who need it is problematic and time consuming.

Commissioner Gottlieb is reorganizing FDA in the hopes of streamlining policymaking.