
Incorporating regulatory requirements into the product life cycle is crucial.

Incorporating regulatory requirements into the product life cycle is crucial.

Follow-on versions of complex biologics require extensive expertise.
BIO's president and CEO provides insight leading up to the 2011 convention.
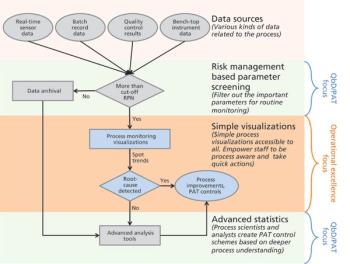
The authors focus on operational excellence in manufacturing of biotechnology therapeutic products in the QbD paradigm.

Industry struggles to curb drug abuse, diversion, and disruptions in supply to ensure access to quality products.
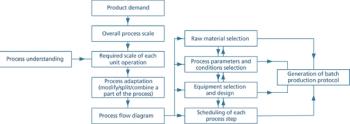
Clear documentation and open communication are essential for effective technology transfer.
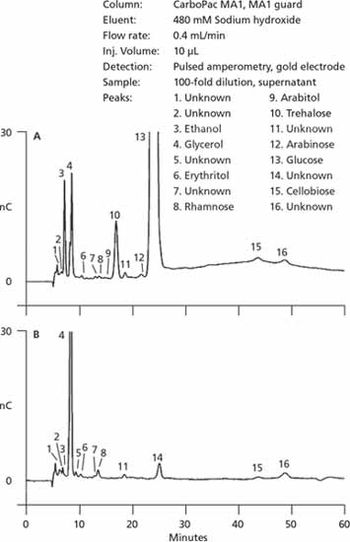
An overview of applications in the analysis of nutrients and metabolites.

A rigorous cost-benefit assessment can help to chart a cost-effective path forward.

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.

Rapid microbial screening provided by contract laboratories can save companies time and money.

FDA, NIH, and industry seek new strategies to spur drug development and promote access to therapies.

Nigeria Looks to Simple Packaging Controls and International Cooperation to Curb Counterfeit Drugs
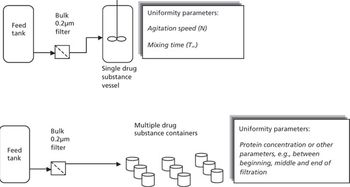
The authors describe considerations and best practices for meeting drug substance uniformity.
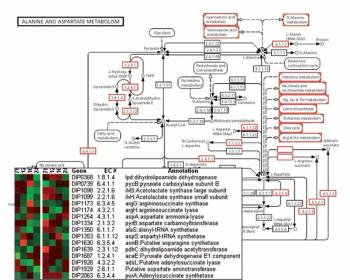
A series of advancements has changed the way bioprocesses are developed and optimized.
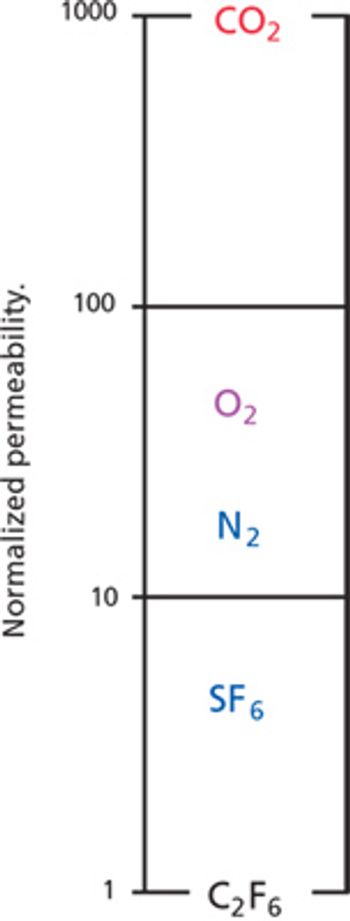
The authors developed a test for defects in filter membranes.

Courts and Congress seek to reshape policies and programs affecting drug costs and access.

Supply-chain analytics can lead to increased profitability.

New supply-chain challenges are forcing companies to act in different ways to secure product safety.

Executive management leadership is essential in the effective implementation of QbD.

US Pharmacopeia develops and improves its class approach for ensuring quality biopharmaceuticals.

The authors present an approach for testing statistical equivalence of two stability profiles.
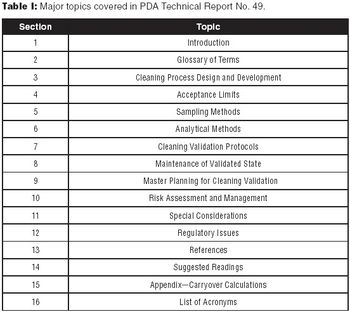
The authors encourage biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.

As drug shortages make headlines, FDA tests the Sentinel safety system and its effect on healthcare.
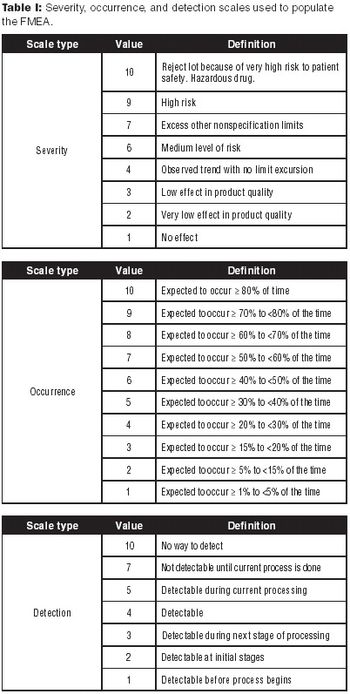
The authors review risk-assessment tools to evaluate product quality.
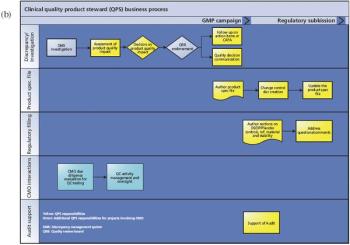
A successful QPS acts as a single point of contact for consistent product quality oversight.