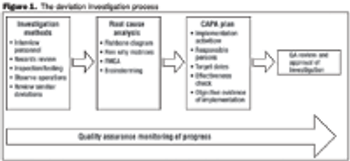
Follow a risk-based approach to maintain a state of control.

Follow a risk-based approach to maintain a state of control.

The 45 comments submitted raised concerns about legacy products and ongoing process monitoring.
A method to evaluate the relative cleanability of new products.

QbD for QA? Try a two-step approach

To stave off quality issues associated with production processes, a centralized and enterprise-wide quality management initiative must be enforced.

A clear oversight framework for biomarker clinical laboratory tests used in personalized medicine is required.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
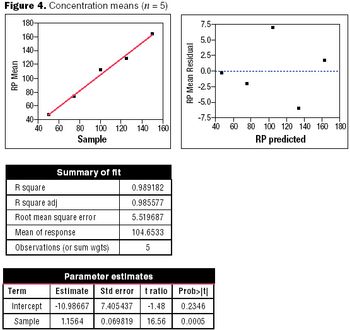
Well-designed experiments can reduce the risk of coming to an incorrect conclusion during a process characterization, assay validation, or process validation study.

A centralized quality control strategy may be the best solution.

Statistical methods for calculating confidence intervals, tolerance intervals, and capability analysis to reduce out-of-specification situations.

Risk mitigation should be a key aspect of any contract manufacturing organization's business strategy.

GMPs in Phase 1 is more important now than ever before.
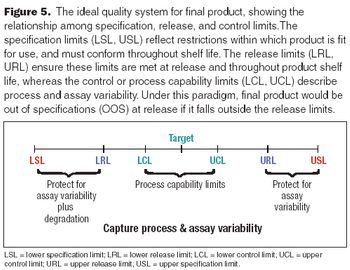
Set limits to provide incentives for process improvements.
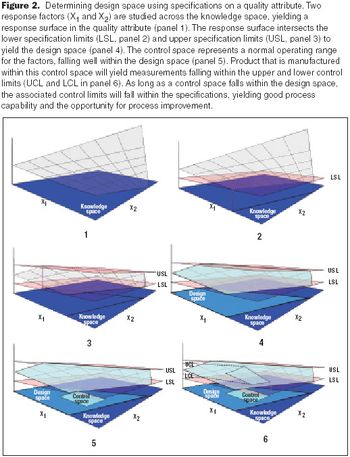
Resolve confusion about measurements.
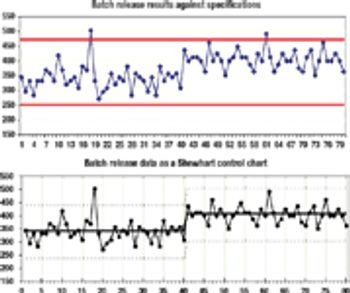
Improving your quality operations by using sound statistical principles.

How to use hypothesis correctly, and understanding the difference between one-sample, two-sample, and z-test.

A staged approach to limits should embrace future capabilities.
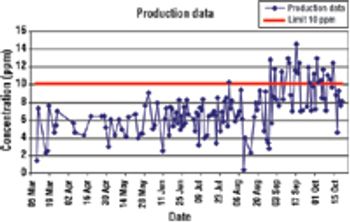
CUSUM charts are easy to create in Excel and can reveal when a change occurred.

There are several considerations to keep in mind when auditing an outsourcing provider.
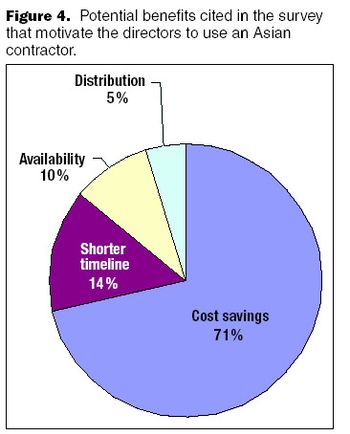
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

Now is a good time for companies to know their suppliers well.
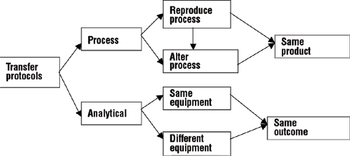
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.
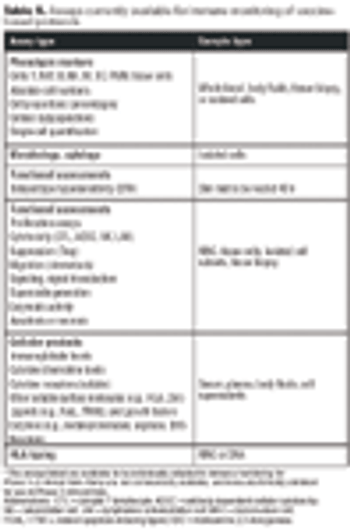
Emerging therapies pose challenges for standardizing QC.

The expanding globalization of the industry poses a challenge for international enforcement.

Need a standard to follow? Just ask, What would Genentech do?