
Have FDA initiatives improved manufacturing quality?

Have FDA initiatives improved manufacturing quality?
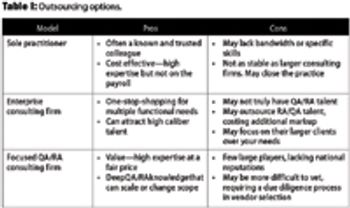
This article examines the options to best match needs and spending for quality and regulatory leadership.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.

Two general chapters on elemental impurities? limits and procedures are to become official Feb. 1, 2013, with implementation proposed for May 1, 2014.

FDA talks about the changing scope of regulatory science.

A recent ISPE guidance provides a baseline for the design of quality laboratory facilities.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.

USP revises labeling requirements for Heparin.

Understanding overall supplier capability versus the critical-to-quality attributes of your product can reduce both risk and cost.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.
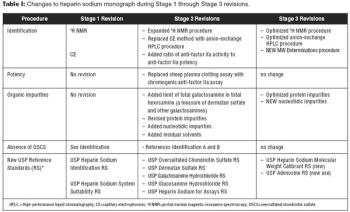
USP optimizes identification tests and impurities procedures.

Anne Marie Dixon of Cleanroom Management Associates, gives us an update on her 1988 article, "Clean Room Management."

USP Hosts Symposium on Science and Standards

Brazil's regulatory health authority, Anvisa, plans to establish quality requirements for locally produced pharmaceutical excipients, Anvisa told BioPharm International.

A one-day sign off for batch records is considered a best practice in the industry.

Recently developed immunoassay technology platforms reduce sample volume requirements and improve cycle times.

Unnecessary analytical testing can lead to unnecessary costs.

Although FDA cannot do anything to stop drugs from being discontinued, it can do something about supply and quality problems that lead to shortages.
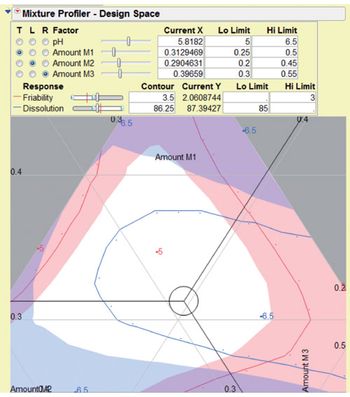
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.

Leading industry collaborators outline top 10 best practices for human error reduction.

The contract provider needs to know as much as the NDA holder.

A closer look at elastomer changeout times provides one example of using industry knowledge to improve operations and cost.

The confluence of science, technology, and regulation will provide our industry with the guidance to move forward.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.
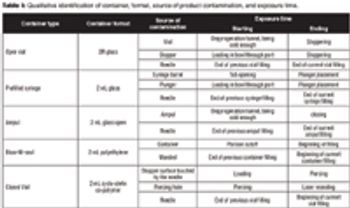
The authors compare the exposure risk from viable particles from the air supply in four well-established aseptic filling technologies.