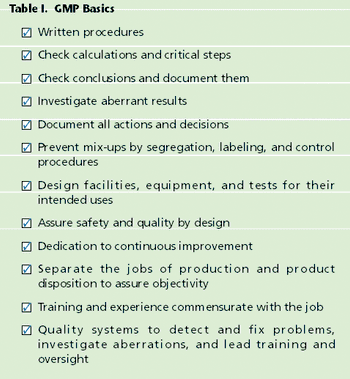
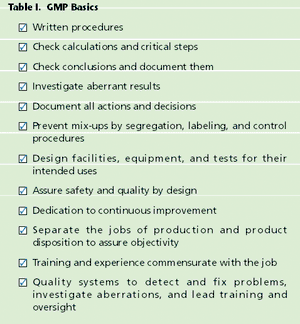
Recent problems with food and pharmaceutical ingredients sourced from China highlight a major disadvantage of our complex international supply chains for food and drug ingredients. A global supply chain offers more opportunities for accidental contamination as well as intentional adulteration and counterfeiting. Sticking to minimal requirements may not be enough.