
In order to institute a GxP mindset across the organization, support and respect for quality systems should come from the top down.

In order to institute a GxP mindset across the organization, support and respect for quality systems should come from the top down.
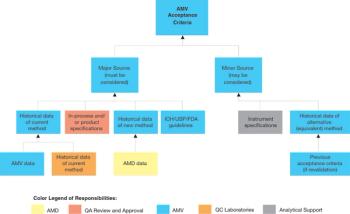
When replacing an existing method, it may be necessary to compensate for a significant difference between the current and new method by adjusting the specifications.

The relationship between "valid" or "suitable and validated" is often overlooked, but there is a high price when "validated" test systems are simply inappropriate.
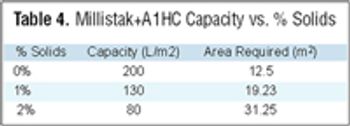
Filtration is one of the most commonly used unit operations in biopharmaceutical manufacturing. Available formats include direct or normal flow filtration (NFF) and cross or tangential flow filtration (TFF). These methods are used for sterilization and virus filtration, depth filtration or ultrafiltration, and diafiltration applications. Some common objectives include:
Networks are part of the compliance picture. Recent FDA warning letters show the agency considers network monitoring and qualification a necessary part of maintaining the security and integrity of electronic records.

The FDA?s risk-based approach to pharmaceutical cGMPs applies to 21 CFR Part 11 enforcement as well. Understanding different methodologies for assessing and managing risk will help you develop and begin to implement a compliance plan.

Protecting the integrity of data is a challenge of 21 CFR Part 11 compliance. Integrity requires records to be complete, intact, and maintained in their original context ? associated with the procedures which were used to create the data.

Bringing different laboratory instruments into compliance takes planning. The key strengths and weaknesses of different levels of control and feedback for analytical instruments and data transfer systems are highlighted in this article.

This article focuses on the front end of qualifying a new raw material from a given supplier. Once qualified, this status must be maintained by periodic review and requalification.

Biotech companies are among the most intensive instrumentation users of any FDA-regulated business. Because of the complex nature of these companies' research and manufacturing, they typically have a larger number of instruments per employee.