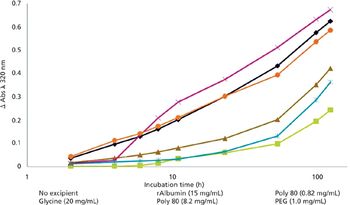
Recombinant albumin can stabilize a drug product and assist in API release.

Recombinant albumin can stabilize a drug product and assist in API release.

Key business considerations when developing biosimilar products virtually.
The authors compare the exposure risk from viable particles from the air supply in four well-established aseptic filling technologies.

Has an approval in oncology reignited interest in the recruitment of the immune system in the fight against disease?

This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."
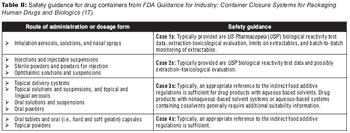
Approaches for risk assessment of extractables and leachables.

The authors describe a novel means to control ice nucleation using a sterile cryogenic ice fog.
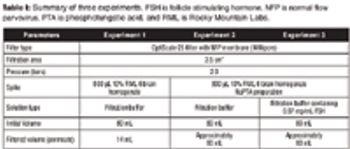
Can a nanofiltration process be leveraged for removal of prions?

Readers react to the economic turmoil of the past year and look longingly forward to 2012.
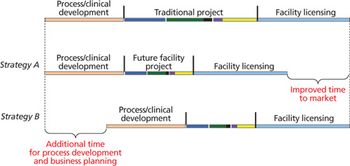
With careful analysis to mitigate risk, disposable technology and process closure can enable adaptable designs and reduced costs.
An evaluation of the technologies needed to develop a safe, effective, and economically efficient vaccine. This article is part of a special section on vaccines.
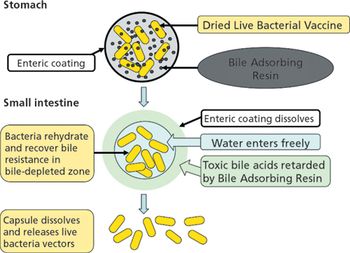
Development of the ideal DNA vaccine requires the optimization of delivery strategies and plasmid vectors.
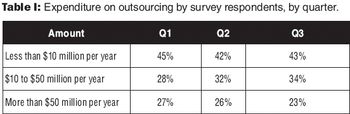
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.

The author reviews the state of downstream processing, including a look at the streamlining of full processes and borrowed technologies.
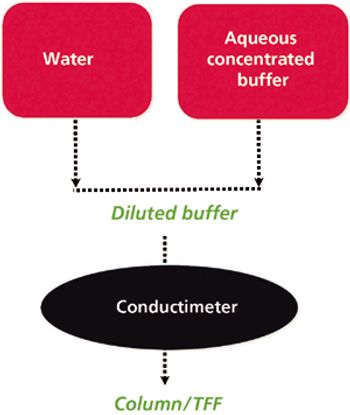
The author describes recent developments to help overcome the downstream-processing bottleneck.
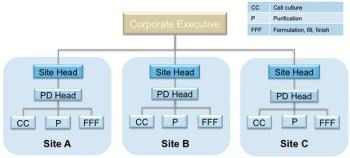
How to strike a balance between site autonomy and global coordination.
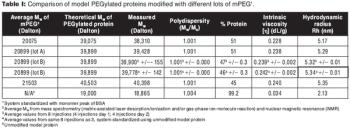
Reliably detecting low amounts of high molecular weight impurities during process development and characterization of biopharmaceutical products.
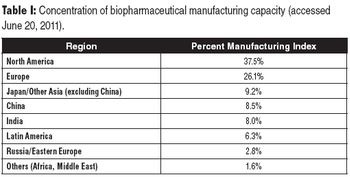
Insights from real-time ranking of global biomanufacturing capabilities.
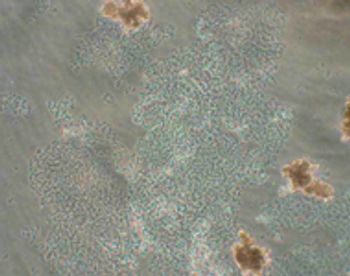
The authors present a case study identifying a contaminant.
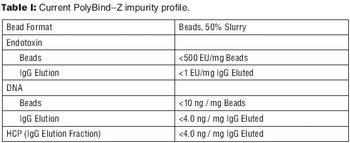
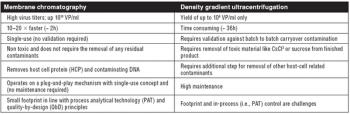
The authors discuss an alternative to traditional Protein A resins.

Rapid microbial screening provided by contract laboratories can save companies time and money.
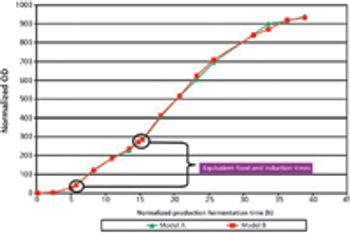
The authors outline qualification procedures for a process-critical piece of equipment.
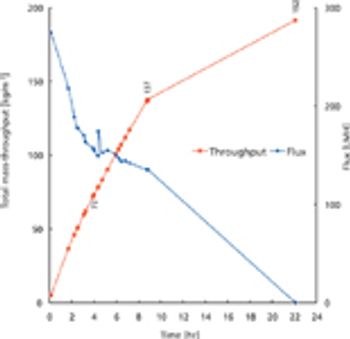
Newer classes of biotherapies will require innovations in processing technology.

A high-performance anion exchange resin performs well compared with membranes. In addition, the resin offers greater flexibility and cost savings.

This summary provides an introduction to our special issue on Separation and Purification.