
FDA seeks industry support for metrics program, emphasizing the surveillance focus.

Chugai Pharmaceuticals, a research-based company headquartered in Tokyo, opens new facility in Berkley Heights, NJ.

The proposed merger of Pfizer and Allergan will create a new top drug maker and cut Pfizer’s tax bill with a headquarters move to Ireland.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.

There’s renewed optimism in the biomedical research community that years of effort finally may begin to pay off for developing cutting-edge gene and cellular treatments for debilitating and life-threatening conditions. Jill Wechsler reports.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.
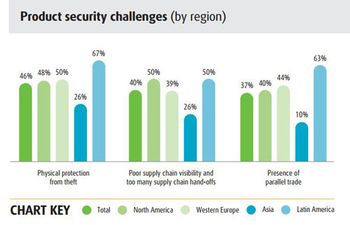
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Thermo Fisher Scientific announced the opening of their new GMP clinical services facility in Singapore.

Pharmaceutical manufacturers should not be protected from antitrust litigation simply because FTC chooses not to pursue a lawsuit, the agency wrote in a recent amicus brief.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

Global spending on medications will continue to rise, and it is expected to increase by $349 billion on a constant-dollar basis by 2020-more than $150 billion more than it increased during the past five years, according to a new IMS Institute for Healthcare Informatics report, Global Medicines Use in 2020: Outlook and Implications. Although the spending increase is large, total spending on medicines is expected to increase at a slower rate than it did over the past five years, when spending on medicines increased approximately 35%.

Merck KGaA announces the completion of the acquisition of Sigma-Aldrich, a St. Louis-based life-sciences and technology company.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.


The new executive director of the European Medicines Agency begins appointment.

A landmark study by the National Institutes of Health determines that Lucentis is highly effective as a treatment for diabetic retinopathy.

FDA grants accelerated approval for Darzalex (daratumumab) for the treatment of multiple myeloma.

A new consortium involving Arecor, FUJIFILM Diosynth Biotechnologies and the Center for Process Innovation will focus on formulation innovation as a way to improve downstream processing and reduce biopharmaceutical cost.

High-dose axalimogene filolisbac immunotherapy will advance to expansion phase.

The University of Pittsburgh partners with biopharmaceutical company, Shire plc, to research rare diseases.

An ABPI report found a lack of quality candidates for high-skilled roles in areas such as bioinformatics, translational medicine, clinical pharmacology, and pathology.

Panelists at the meeting will focus on clinical trial design, immunogenicity, and enhancing implementation plans for administering already-licensed vaccines to this patient population.

As a result of the acquisition, Merck will have access to Harrisvaccine’s proprietary RNA Particle technology production platform.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.

A study and new index shows that only 65% of clinical trials are being registered and reported on. Nearly half of the new drugs approved by FDA in 2012 had one Phase II or III trial that was not disclosed. Regulatory ambiguities, mergers, and lack of enforcement action may be to blame

Lawsuit alleges birth control packaging error led to 113 unwanted pregnancies.

Connected Services program will link cGMP-manufacturing services with supply chain technology management solution.