
Rafe Swan/Getty Images; Dan WardIrreproducible preclinical research is a global, expensive, and well-recognized problem that contributes to delays a

Rafe Swan/Getty Images; Dan WardIrreproducible preclinical research is a global, expensive, and well-recognized problem that contributes to delays a
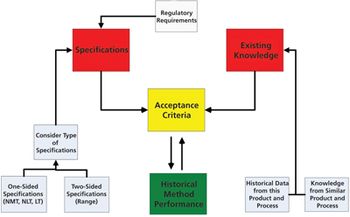
The authors present the results of a survey of biologics manufacturers to evaluate how these manufacturers transfer analytical methods.
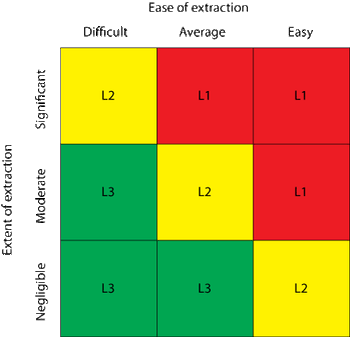
The author explores a dual-supplier sourcing strategy for single-use products and its ability to mitigate business continuity risk.

Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.
Dynamic light scattering techniques can monitor viruses and virus-like particles in their native state.

In the development of biopharmaceuticals and pharmaceuticals, the line is blurring.
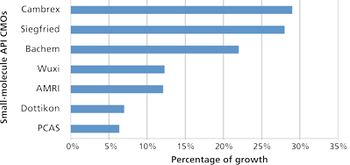
Despite emergence of biologics, small-molecule APIs benefit from industry growth.
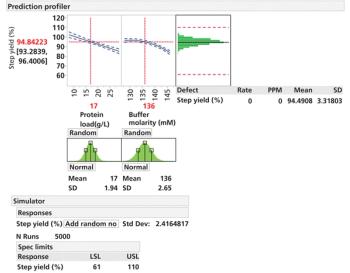
An approach to small-model generation and calibrating small-scale models to reliably predict performance at scale is presented.

Click the title above to open the BioPharm International October 2015 issue in an interactive PDF format.

China’s emergence as a significant commercial market is forcing manufacturers to re-evaluate their overall business model.