
Structures and Manufacturing Support

Structures and Manufacturing Support

PDUFA renewal legislation sets stage for new policies affecting revenue, research, and oversight.

The author reviews the state of downstream processing, including a look at the streamlining of full processes and borrowed technologies.

Why SOPs are rarely followed, often cited, and in great need of follow-through.
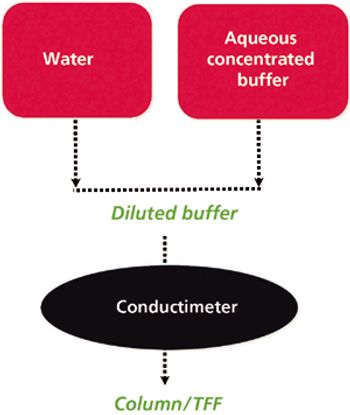
The author describes recent developments to help overcome the downstream-processing bottleneck.
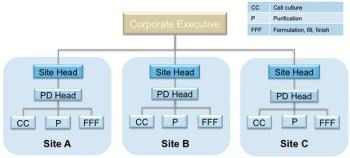
How to strike a balance between site autonomy and global coordination.

A report commissioned by FDA evaluates the QbD paradigm.

The EU debt crisis portends of possible negative repercussions for the dose CMO industry.

FDA weighs multiple views regarding the Biologics Price Competition and Innovation Act.