
Celebrating 25 Years of BioPharma Innovation

Celebrating 25 Years of BioPharma Innovation

BIO is calling for a more patient-centric approach to user-fee reauthorization.

Implementing quality by design makes the determination of quality metrics across CMOs and sponsors essential.
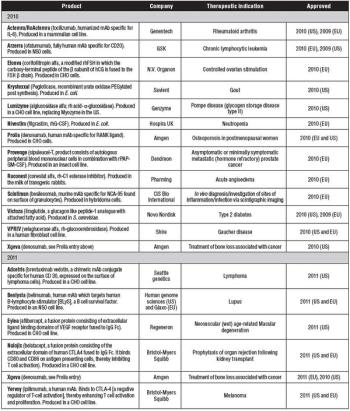
A review of new biologic drug approvals over the years, featuring highlights from 2010 and 2011.

Leading industry collaborators outline top 10 best practices for human error reduction.

In a special anniversary interview, Washington Editor Jill Wechsler speaks with with FDA Deputy Commissioner Deborah Autor about where the agency is headed.

The contract provider needs to know as much as the NDA holder.

Mike Clayman, CEO of Flexion Therapeutics, talks about his company's strategy to focus on a single therapeutic area.

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

A review of key industry shifts and promises for the future.
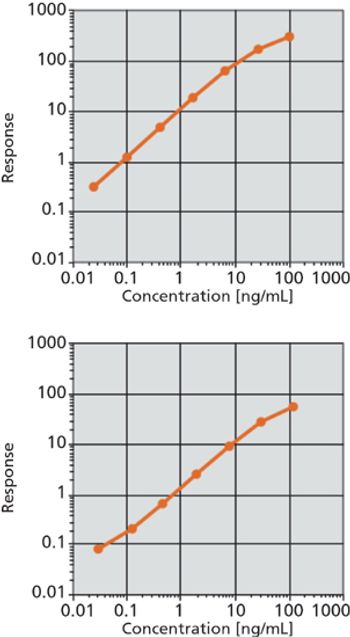
The authors discuss a new, rapid immunoassay for the detection of biomarkers.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Industry experts discuss significant achievements. Plus: What's in store for the future.

In this online extra, Board Members comment on major industry changes over the past few decades.