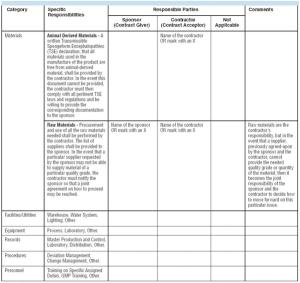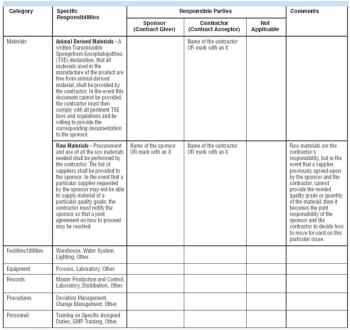
During the past several years in the pharmaceutical and biopharmaceutical industries, conflicts and misunderstandings have arisen between companies and their contractors. Too often, productive working relationships have crumbled, resulting in expensive production delays with companies and contractors squabbling over their roles and responsibilities. Such conflicts may have their roots in the lack of a sound quality agreement (QAG). QAGs that clearly delineate good manufacturing practice (GMP) responsibilities between a sponsor and a contractor can help companies and their contractors avoid certain conflicts.