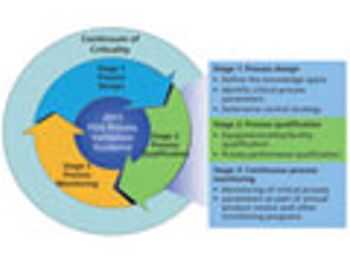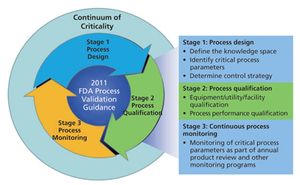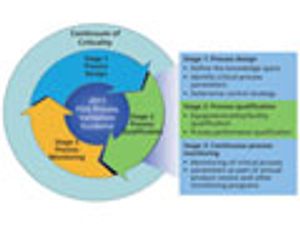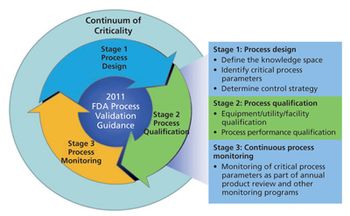
This series presents a practical roadmap in three parts that applies scientific knowledge, risk analysis, experimental data, and process monitoring throughout the three stages of the process validation lifecycle. In Parts I and II, risk analysis and process characterization studies were used to assign criticality risk levels to critical quality attributes and critical process parameters, and the concept of a continuum of criticality was established. In Part III, the author applies the continuum of criticality to develop the process control strategy and move through Stages 2 and 3 of the new process validation lifecycle.