
Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.
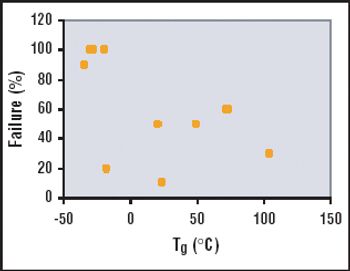
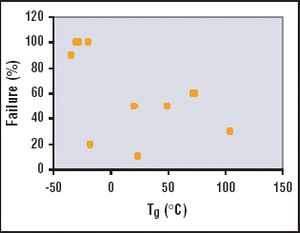
The adoption of single-use containers in the biopharmaceutical industry is becoming more frequent as the popularity and availability of the technologies increase. The choice of a solution for storage in single-use containers clearly depends on the application and the inherent risks associated with the application. A "one fits all" single-use system cannot respond to all the requirements of a particular step in a biopharmaceutical process, much less to all the steps of a process. The needs of an application will lead to very specific single-use solutions.
Published: November 2nd 2006 | Updated:

Published: June 15th 2018 | Updated: