
Awareness of recently implemented—or ongoing—advances by the pharmacopoeias can help biotherapeutic manufacturers remain compliant with current requirements.
Joseph A. Albanese is the director of Analytical Strategy and Compliance at Janssen Research and Development, LLC.

Awareness of recently implemented—or ongoing—advances by the pharmacopoeias can help biotherapeutic manufacturers remain compliant with current requirements.

Processes, people, and tools are necessary to comply with the pharmacopoeia and approved drug product registrations.

This article details the more operational aspects of monograph submissions, answering the question of how to participate.
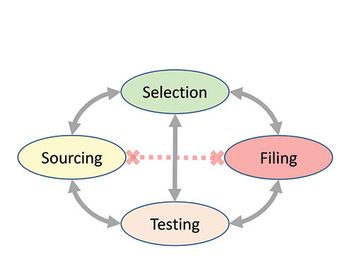
The authors present a case study with raw materials and excipients, where a consistent, cross-functional approach is needed to ensure the appropriate selection, sourcing, testing, and filing of the materials used to manufacture bio/pharmaceutical products in a global environment, ensuring compliance with applicable compendial and regulatory requirements.

This article explores another proactive advocacy approach that a bio/pharmaceutical company may take through participation in the development of new and revised monographs in the various pharmacopoeias.

This series is intended to address the challenges for the industry to comply with pharmacopoeial requirements. This article returns to this important topic with a case study at the intersection of monograph development and compliance.

This article summarizes all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world. This look into what is on the horizon is important to help companies prepare for the inevitable changes and ensure the continued supply of quality medicines to patients globally.

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article returns to the topic of complying with pharmacopoeial requirements with a case study at the intersection of monograph development and compliance.

This article details the more operational aspects of monograph submissions, answering the question of how to participate.

This case study is based on the experience of one of the authors but is applicable to all companies across the broader industry, illustrating the potentially surprising point that some compliance difficulties may be of the company’s own making.

This article explores another proactive advocacy approach that a bio/pharmaceutical company may take through participation in the development of new and revised monographs in the various pharmacopoeias.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article presents a case study at the intersection of monograph development and compliance.

The revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world are described.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An understanding of global and national pharmacopoeias is crucial to understanding change processes and access to different markets.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.
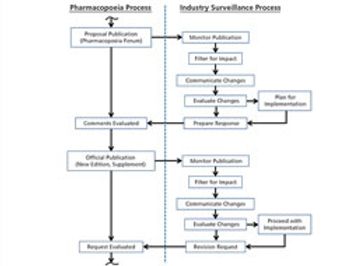
The process used to monitor and participate in pharmacopoeial changes is described.

The authors take a closer look at these ongoing efforts to harmonize compendial standards, with perspective that may be helpful in considering the future direction of pharmacopoeias.

Pharmacopoeia harmonization provides better support for global regulatory agencies and addresses the global nature of bio/pharmaceutical manufacturing and supply.

This article examines the history and evolution of the pharmacopoeias and the particular challenges that must be overcome to achieve harmonization among the pharmacopoeias.

This article provides an end-to-end compendial framework to understand why compliance with pharmacopoeia standards is challenging.

This article provides the legal and regulatory basis for pharmacopoeia compliance and illustrates pharmacopoeia impact throughout the drug product lifecycle.

In this series of articles, the authors provide an understanding about the need for pharmacopoeia compliance and practical guidance to assist those who perform this work.

In this series of articles, the authors provide an understanding about the need for pharmacopoeia compliance and practical guidance to assist those who perform this work.

Published: September 15th 2019 | Updated:

Published: September 15th 2019 | Updated:

Published: September 15th 2019 | Updated:

Published: September 15th 2019 | Updated:

Published: September 15th 2019 | Updated:

Published: September 15th 2019 | Updated: