
Interview with David Radspinner, director of global marketing & customer applications, bioprocess production at Thermo Fisher Scientific.


Interview with David Radspinner, director of global marketing & customer applications, bioprocess production at Thermo Fisher Scientific.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.

As the use of disposable bioprocessing equipment has increased, a new question is gaining prominence: What is the best way to dispose of the equipment after use?

SciLog, Inc. (Middleton, WI), a privately held company that designs and manufactures computer-controlled bioprocessing equipment, has announced the signing of a patent licensing agreement with GE Healthcare (Somerset, NJ).
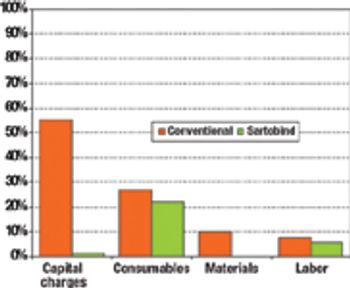
The current focus on cost-of-goods (COGS) models is underplaying the benefits of disposables technology in biopharmaceutical manufacturing. The best method for accounting for the benefits of reduced and delayed capital expenditures is through the use of NPV analysis.

DSM Biologics (Heerlen, the Netherlands), and Upfront Chromatography A/S (Copenhagen, Denmark) have announced a collaboration to optimize Upfront’s new, fully disposable chromatography system for use with DSM's proprietary manufacturing technology.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted

Saint-Gobain Performance Plastics Corporation (Aurora, OH) has acquired the assets of J & J Scientific Products, Inc. (Tampa, FL), a manufacturer of disposable plastic products for the biopharmaceutical market.

Two case studies illustrate a systematic approach.

The use of disposables has changed significantly in the biopharmaceutical industry.

The automated NucleoCounter from New Brunswick Scientific use disposable cassettes, pre-filled with propidium iodide for accurately counting cells.
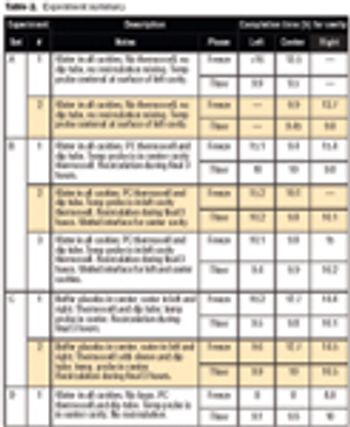
A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.
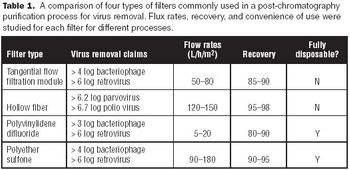
One of the challenges of adopting single-use technology is that not all cell lines are compatible with disposable bioreactors.
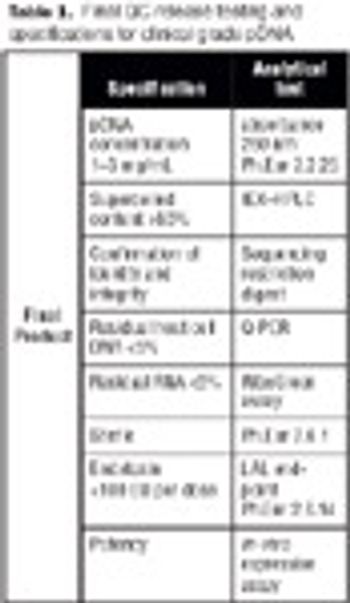
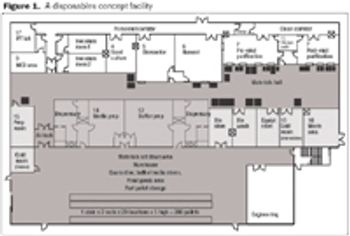
Disposables are increasingly being used in the manufacture of biopharmaceuticals. This article describes the design of a fully disposable process for the cGMP manufacture of clinical trial grade plasmid DNA. It addresses the rationale for implementing such a process with respect to the manufacture of patient-specific plasmid DNA vaccines for the treatment of leukemia. The process incorporates a number of disposable technologies, which are simple to use and thus reduce the need for investment in expensive equipment and cleaning validation.
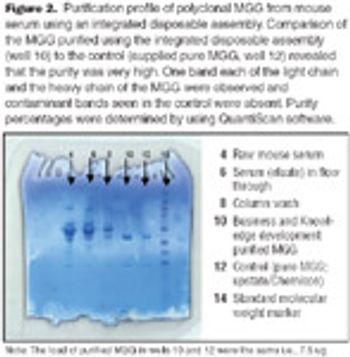
Comparison of the integrated assembly purified MGG to the control revealed that the purity if the MGG was very high.
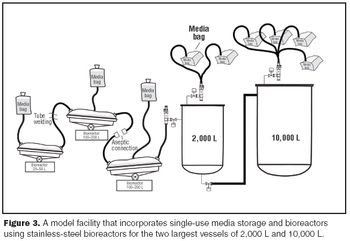
Single-use systems reduce maintenance and capital expense by eliminating expensive vessels, valves, and sanitary piping assemblies.

The BIOSTAT Cultibag RM is a disposable bioreactor that combines rocking motion mixing technology with Sartorius engineered control capabilities.
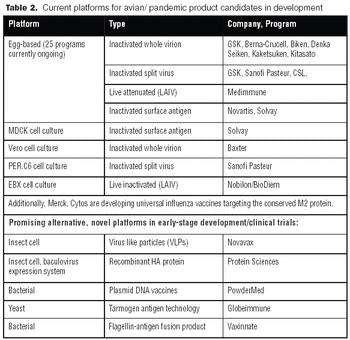
For pandemic vaccine processing, single-use filter cartridges and membrane chromatography technologies could offer significant time- and cost-reduction advantages.

The recent growth in the vaccine market has led to renewed interest in using adherent human cell lines for vaccine production. Traditionally, small-scale adherent cell line production has been carried out in roller bottles or T-flasks. Over the past few years, however, a number of companies have found multi-tray disposable bioreactors an effective method for producing high-quality drug products using adherent cells. These disposable, expandable systems have also facilitated scale up from laboratory to clinical-scale.

Vaccines against strains originating from avian flu may achieve poor yields in egg-based systems. Consequently, both public and private interest in alternative systems is high.

The BioWelder and BioSealer from Sartorius (www.sartorius.com) are suitable for connecting or disconnecting thermoplastic tubing in biopharmaceutical manufacturing processes.

Today, most disposables are used for process development and clinical-scale manufacturing. Substantial growth in disposables usage may not occur until disposables are incorporated into the production of licensed products at commercial scale.

Millipore Corporation's disposable mixer is designed to help companies mix pharmaceutical ingredients and prepare cell culture media.

Energy input is affected by rocking the chamber back and forth, generating a fluid movement in the cell culture and medium.