
While the severity of capacity problems related to downstream processing appears to have eased, it continues to be a problem and chromatography columns are the most frequent culprits.

While the severity of capacity problems related to downstream processing appears to have eased, it continues to be a problem and chromatography columns are the most frequent culprits.
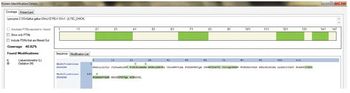
A simple, sensitive, and universal platform for identification and quantification of trace-level protein impurities in biotherapeutics is described.

Pharma eyes biologics production in Brazil as the government begins to recognize the potential of these drugs.

Updates on user fees, heparin supply concerns, orphan drug incentives, REMS updates, reference standards for proteins, and patent settlements.

Mike Jenkins, general manager of Catalent Biologics discusses the evolving landscape of the biologics market and hurdles faced in the development and manufacture of these innovative products.

Establishing a well-defined training program is a crucial activity for any biopharmaceutical organization.

Sartorius Stedim Biotech's Sartoguard NF prefilter series features a combination of high-performance PES and innovative nano-fleece technology.

Tosoh Bioscience's two ion exchange chromatography resins are designed for the high-resolution purification of proteins, peptides, and oligonucleotides.

The aim of the European Falsified Medicines Directive is to improve the quality of imported APIs, but does the pain now outweigh the gain?

Restricted access barrier systems (RABS) maximize product control but minimize operator interaction in aseptic manufacturing.

Are strategic partnerships in clinical research a model for CMC services?

Novasep's Sius single-use tangential flow filtration skid offers a 100% single-use TFF solution.

New FDA supply chain policies aim to strengthen inspection and oversight processes.

As the complex requirements of manufacturing biologics are manifold, it is important that biomanufacturing companies adopt quality-by-design principles.
Review the importance of characterization studies during biosimilars development and related analytical methods.

PDA/FDA regulatory conference promotes a commitment to quality.
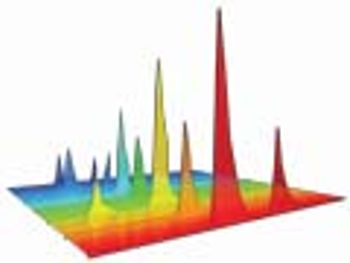
Knowledge of product or process acceptance criterion is crucial in design space.

Click the title above to open the BioPharm International September 2013 issue in an interactive PDF format.

Trends in BioPharma