The corruption of China's food and drug sectors is not limited to one man. Likewise, real reform will require the efforts of many.
The corruption of China's food and drug sectors is not limited to one man. Likewise, real reform will require the efforts of many.

Understanding the end-to-end management of chemistry, manufacturing, and controls (CMC) resources provides the opportunity to enhance long-term planning, leverage development options, manage resource trade offs, and track progress against plans. The goal is to improve the pharmaceutical development process to deliver the pipeline. This article provides an overview of the organizational structure of Process Research and Development (PR&D) and the CMC teams at Genentech; the alignment of resources based on CMC contracts, process development activity maps and project resource plans; and the business economic analysis for evaluating development options.

When you start out, keep your small-cap exposure to no more than 10% of your holdings, and gradually increase.

The main testing and regulatory provisions of the FOB legislation reflect multiple trade-offs between the demands of innovators and generics firms.
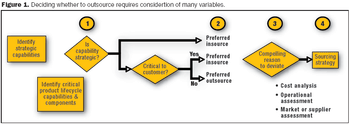
Companies need to avoid operating in a manner that is inconsistent with the priorities established in the strategic sourcing decision.
Drug delivery technologies have the potential to enable drug candidates with poor pharmaceutical or biopharmaceutical properties, both for macromolecule and traditional compounds. While there have been many success stories to date, the future offers even more promise. In this article, the author surveys the top ten areas in drug delivery looking forward.

The mounting threats of pandemic influenza, bioterrorism, and emerging infectious diseases continue to be the focus of research programs and funding initiatives, not only within governmental agencies, but also universities, private research firms, and commercial manufacturing entities. With all of these efforts, however, the question of manufacturing capacity and the ability to respond to pandemic and emerging threats continues to be a major concern.
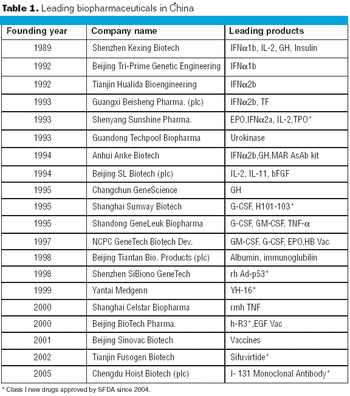
In 2006, China exported a total of $890 million in biologics to other countries-a 30.61% increase, compared with the previous year.

Companies in the biotech industry typically require one or several partners as they complete the product development cycle.