
Pharma and biotech companies, with the rest of the healthcare industry, must face change.

Pharma and biotech companies, with the rest of the healthcare industry, must face change.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.

European governments are under pressure to take regulatory action, but solving the problem of medicine shortages is not as straightforward as it seems.
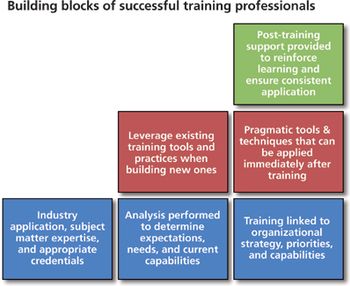
Five key attributes to look for in a biopharm training partner.

Brazil offers opportunities and challenges for global pharmaceutical companies.

The industry may not be ready for India and China as regulatory issues emerge.
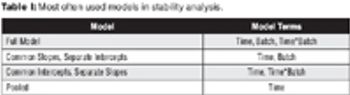
The author discusses the need for stability analysis.

An analytical method assesses the effects of media condition on cultured antibodies.

Increased manufacturer outsourcing requires clear policies and written agreements with CMOs.

Biotech companies raise most money in 13 years.
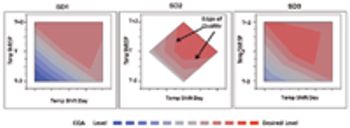
Traditional project decision-making vs. a QbD approach.

Click the title above to open the BioPharm International July 2013 issue in an interactive PDF format.