
Using Blow-Fill-Seal Technology

Using Blow-Fill-Seal Technology

Incorporating regulatory requirements into the product life cycle is crucial.
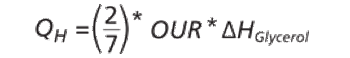
The authors describe challenges faced in transfer and scale-up of a fermentation process.

Which route will we take to arrive at a national stem-cell policy?

Plasmid DNA-encoding proteins offer many advantages, which are now being used in clinical trials.

Strong pipelines, approvals, and deals drive up market cap.
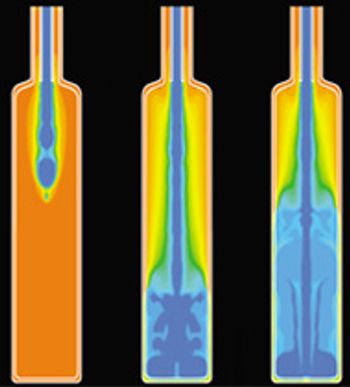
The authors give special consideration factors affecting blow–fill–seal technology.

Better strategies and practices in sourcing and procurement can contribute to the bottom line.

Follow-on versions of complex biologics require extensive expertise.