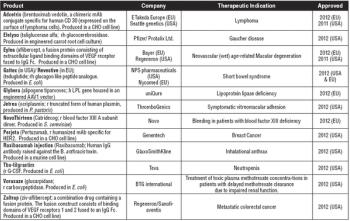
First gene therapy and plant-based expression vector products approved in 2012.

First gene therapy and plant-based expression vector products approved in 2012.
The author outlines the need for custom-cell products.

Benchmarking can be a useful tool to improve manufacturing practices.

An innovative approach to capacity management.

Have FDA initiatives improved manufacturing quality?

Latin America's diverse growing market seeks regulatory harmonization.

The authors propose a roadmap for analytical lifecycle development.

Opioid abuse generates calls for efforts to curb distribution.

Sound policies are needed to govern the substitution of interchangeable biologics.

Early-stage companies are finding alternatives to venture capital.

Applications of ZFN technology in biopharmaceutical cell-line engineering.
Ontario-area scientists discuss approaches to development of stem-cell therapies.

EU authorities are stepping up their efforts to incorporate QbD principles.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

The Thai government is ramping up efforts to promote and develop the biotechnology sector in a bid to enhance its global competitiveness.

Click the title above to open the BioPharm International April 2013 issue in an interactive PDF format.