Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.
Consider automation early in the rollout of clinical translation and scale up of clinical-trial protocols.

FDA approves Novartis’ CAR-T therapy, marking the first time a cell therapy based on gene transfer has been approved in the United States for any indication.

FDA noted in a recent inspection that US Stem Cell Clinic was processing and administering stem cell treatments that were neither reviewed nor approved by the agency.

Pfizer will invest $100 million to expand its manufacturing facilities in Sanford, North Carolina.

Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

New patent for automated cell processing technology provides commercially viable automated CMC solution for developing CAR-T cell therapies.

FDA advisory committee has recommended for approval Novartis CAR-T cell therapy CTL019 for the treatment of relapsed and refractory B-cell acute lymphoblastic leukemia in pediatric and young adult patients.

Perfusion processes can attractive for biologics drug manufacturing; however, obstacles remain.
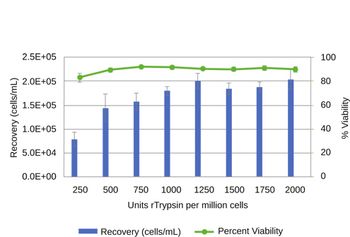
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.

Lonza revealed it received a warning letter from FDA for its cell therapy manufacturing plant.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

The company has signed two separate agreements with the Agency for Science, Technology, and Research (A*STAR) and the Nanyang Technological University (NTU) to progress its therapeutic stem cell pipeline.

In a FDAVoice blog post, CBER Director Peter Marks discusses the new designation for cell therapies that treat life-threatening diseases.

Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

The company announced that it would now be offering a portfolio of fresh and cryopreserved human and animal hepatocytes for ADME-Tox testing.

CellGenix will add R&D, production, and warehouse space in Freiburg, Germany for GMP-grade raw materials for cell therapy, gene-therapy, and tissue-engineered products.

This three-year partnership will explore and identify new tools and methods to modify and optimize the Chinese hamster ovary (CHO) cell line performance.

Patients with relapsing-remitting multiple sclerosis (MS) who are treated with currently available disease-modifying drugs (DMARDs) usually experience disease reactivation within the first five years of treatment follow-up. Many of the available treatments for MS only confer complete control of disease activity in a small percentage of patients.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.

Researchers from Cellectis investigate how external signals in the tumor microenvironment could control the cell-surface expression and specificity of engineered CARs.

Language surrounding regenerative medicine and the REGROW Act appeared back into the 21st Century Cures Act right before it passed. What will this mean for the controversial testing and marketing of stem-cell therapies?

UK-based Orchard Therapeutics and PharmaCell ally to support clinical trials and commercialization of Orchard’s ex-vivo gene therapies.