
EMa and FDA are seeking potential candidate companies for a joint GMP inspection pilot program.

EMa and FDA are seeking potential candidate companies for a joint GMP inspection pilot program.

While not yet finalized and adopted, ICH Q10 represents some of the most current thinking with respect to pharmaceuticals manufacturing and control.
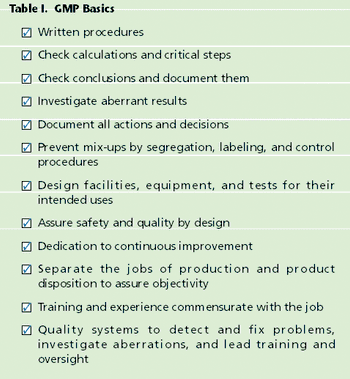
What are current good manufacturing practices (cGMPs)? Where did they come from? What are the actual "practices" described in the Code of Federal Regulations, 21 CFR. If you are new to the pharmaceutical or biotechnology industries, you may enter your first "GMP Training" session without much context or perspective. A set of arcane rules is presented; you were never taught these in science classes.

The European Union requires final container testing of US-manufactured biopharmaceutical products to be performed in Europe for release into the European market. Similarly, but less strictly enforced, the US requires final container testing in the US for European-manufactured biopharmaceutical products before release.