
Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Europe prepares for inclusion of Croatia in EMA activities.

The EMA's Committee for Medicinal Products for Human Use has recommended granting of marketing authorizations for the first two monoclonal antibody biosimilars.

EMA?s revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

EMA clarifies biosimilars guidelines.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

EU authorities are stepping up their efforts to incorporate QbD principles.

The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended Sanofi's six-in-one pediatric vaccine for marketing authorization.

The European Commission claims that Johnson & Johnson and Novartis may have breached European antitrust rules.

The management board of the European Medicines Agency (EMA) has endorsed the agency?s work programme and budget for 2013, which includes a budget of EUR231.6 million, a slight increase over 2012.

The Court of Justice of the European Union has dismissed an appeal by AstraZeneca concerning a 2005 decision that found the company guilty of abusing its dominant position in the marketplace.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

NIBRT's Jayne Telford provides an overview of biopharmaceutical analytics and their accompanying qualification and validation steps.
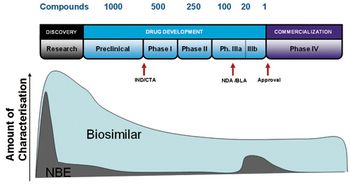
Understanding opportunities and challenges across all major phases of development.

The European Medicines Agency (EMA) has abolished its Cell-based Product Working Party (CPWP) and Gene Therapy Working Party (GTWP), with the aim of improving efficiencies and optimising the use of available expertise.

The European Generic Medicines Association (EGA) has raised concerns about the potential fees to be charged by the European Medicines Agency for pharmacovigilance activities

The European Medicines Agency has launched a public consultation concerning its inventory of paediatric medicines with the aim of highlighting where further R&D efforts are required. The consultation is the first of its kind in this area.

The European Medicines Agency has recommended that the anticancer medicine DepoCyte be recalled from EU countries following the discovery of manufacturing deficiencies at Pacira Pharmaceuticals' San Diego site.

The European Medicines Agency (EMA) will soon be phasing out follow-up measures to marketing authorisations in place of a new system of classification that will be introduced in a stepwise manner.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European-Union customs in 2011.

The European Medicines Agency has launched an investigation into Roche after an inspection found that thousands of potential safety reports, including 15161 deaths, connected to Roche medicines had not been evaluated to determine whether they should be reported to regulators as adverse drug reactions.

The European Medicines Agency's Committee for Orphan Medicinal Products made recommendations for nine orphan drug designations during its June 2012 meeting. Included in COMP's recommendations were four designation applications for rare forms of lipodystrophy.
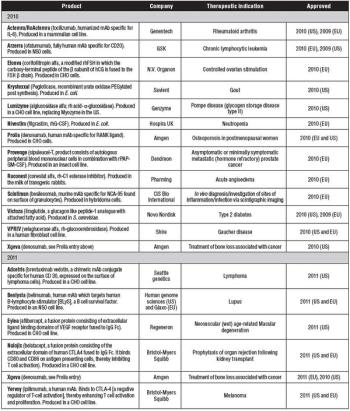
A review of new biologic drug approvals over the years, featuring highlights from 2010 and 2011.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has welcomed the launch of a EUR 223.7-million ($276.5 million) program to tackle antimicrobial resistance and speed up the development of new antibiotics.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has formally adopted a memorandum of understanding (MoU) with key partners for a harmonised, European system for medicines verification.