
How to handle and respond to a consent decree.

How to handle and respond to a consent decree.

FDA has created a dedicated cadre of foreign drug investigators and established permanent offices worldwide.

Soaring opioid use creates challenges for new drug development and supply-chain control.

A closer look at elastomer changeout times provides one example of using industry knowledge to improve operations and cost.

This month, we rewind to an article titled "Good Manufacturing Practices Training."

The confluence of science, technology, and regulation will provide our industry with the guidance to move forward.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.

Challenges of vaccine development include regulatory, technical, and manufacturing hurdles in translating a vaccine candidate into a commercial product.

Does global development have to entail multiple comparability studies?

Social media use raises questions about applying old standards to new information technology.

Key business considerations when developing biosimilar products virtually.

Has the long-awaited guidance answered all of the industry's questions?
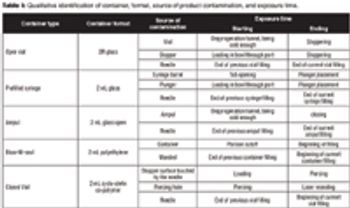
The authors compare the exposure risk from viable particles from the air supply in four well-established aseptic filling technologies.

Focusing on how risk affects the entire organization can improve the business bottom line.

Key technical considerations when developing a clinical project in the biotech world.

New educational programs are key to the industry's future and to safe, available drugs.
Strategies for transfer of the manufacturing process.
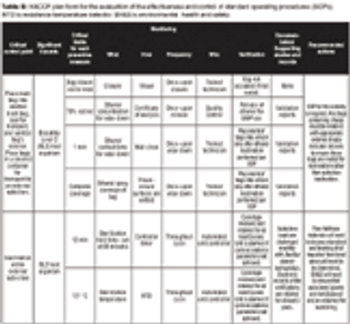
A Risk-Management Case Study.
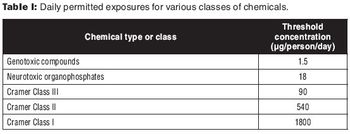
This article is the second in a two-part series on extractables and leachables.

Introducing a new way to think about sharing information in a patent-driven industry.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

The benefits of harmonization may be on industry's wish list, but buying into change is another story.

Government plans require investment, partnership, and industry collaboration.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.