
AptarGroup’s Landfill Free Certification program addresses the issue of waste production from pharmaceutical manufacturing processes by prompting its facilities to reduce and reuse operation waste.

AptarGroup’s Landfill Free Certification program addresses the issue of waste production from pharmaceutical manufacturing processes by prompting its facilities to reduce and reuse operation waste.

The companies aim to discover and develop locked nucleic acid oligonucleotides as orally available therapies for treating inflammatory bowel diseases.

The companies aim to develop a potential zinc finger protein transcription factor-based gene therapy for treating Lou Gehrig’s disease.

FDA Commissioner Gottlieb expects that the saline and amino acid drug shortages from Puerto Rican manufacturing facilities will improve in the early part of 2018.

The companies will collaborate on the development of vaccines to expand GeoVax’s cancer immunotherapy program.

The IFPAC annual meeting on advancing the understanding and control of manufacturing processes using process analytical technology will be held Feb. 11-14, 2018.

The contract manufacturing company has completed construction on a new aseptic fill/finish facility in Wuxi, China.

Proper selection and installation optimizes fluid system performance.

The Wilden Quattroflow QF10k size pump from PSG, a Dover company, has been designed to fill the gap between the existing QF4400/5050 and QF20k pump sizes.
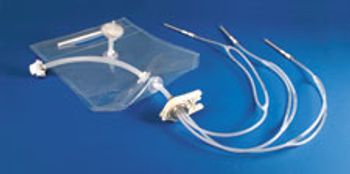
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.

Pharmaceutical companies and contract manufacturing organizations report lack of readiness for the November 2018 US Drug Supply Chain Security Act serialization deadline.

A review of 2017 advancements from the Open-SCS working group with Charlie Gifford, group technical director.

New technological advancements seek to improve pharmaceutical process monitoring and control.

Open standards based on GAMP and GS1 will soon be released and more companies are moving beyond basic compliance.
Advances in TFF and single-use systems help advance UF/DF on the continuous processing path.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).
Recent investments show expansion activity in cell culture facilities.

The biotechnology company has filed for FDA approval of a new plasma manufacturing facility in Covington, GA, to support its immunology franchise.

The recall was initiated due to a product complaint in which white particulate matter, identified as mold, was discovered in a flexible bag from one batch.

FDA has approved a new gene therapy for treating patients born with a rare, inherited vision loss.

MilliporeSigma will collaborate with IPS and G-CON to offer end-to-end, turnkey, modular MAb manufacturing.

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

Sanofi Genzyme and its partner, Alnylam Pharmaceuticals, has filed a marketing authorization application with EMA for an investigational RNAi therapeutic for treating a genetic-based disease.

The company has completed development of a first-generation production process for its chimeric antigen receptor regulatory T cell product portfolio and is selecting a CMO for clinical supply.