
An analysis of current and upcoming industry challenges.

An analysis of current and upcoming industry challenges.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.

How to implement a risk-based approach to eliminating viruses.
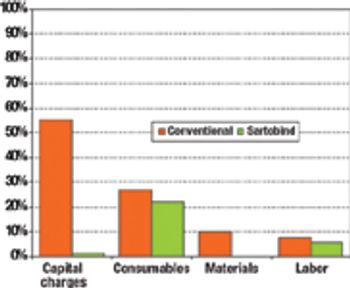
The current focus on cost-of-goods (COGS) models is underplaying the benefits of disposables technology in biopharmaceutical manufacturing. The best method for accounting for the benefits of reduced and delayed capital expenditures is through the use of NPV analysis.
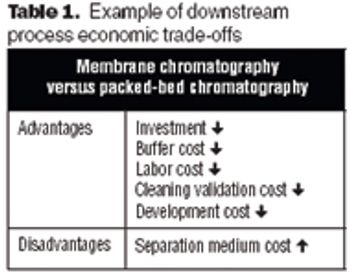
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

Cleaning validation is a critical consideration in the pharmaceutical industry. Inadequate cleaning can result in contamination of drug products with bacteria, endotoxins, active pharmaceuticals from previous batch runs, and cleaning solution residues. Such contaminants must be reduced to safe levels, both for regulatory approval and to ensure patient safety.

By Rajiv Nayar and Mark C. Manning, HTD Biosystms, Inc., pp. 20-28. Outsourcing is often considered a way to expedite drug development, but other options exist for companies that don't choose it yy} or that run up against the capacity shortage. The resources devoted to speeding up the drug discovery process led to combinatorial libraries, high-throughput screening, proteomics, and genomics. Now the same types of innovation can be applied to drug development to prevent valuable lead compounds from sitting idle on the shelf.